Increasing the yield and quality of natural indigo cake using low-cost calcium hydroxide
DOI:
https://doi.org/10.55674/cs.v18i1.263003Keywords:
Lime kiln, Eggshells, Golden apple snail shells, Indigo cake, Indigo-dyed fabricAbstract
This research aimed to improve the yield and quality of natural indigo cake by using low-cost calcium hydroxide produced from household waste, specifically golden apple snail shells and eggshells, as alternatives to commercial lime. Quicklime (CaO) was prepared by calcination in a custom-designed 50-liter vertical kiln at ≈ 900 °C for 4 hours, followed by slaking to obtain hydrated lime (Ca(OH)₂). XRD, SEM, and FT-IR analyses confirmed that both limes consisted primarily of Ca(OH)₂, with snail shell lime showing higher purity and rougher particle surfaces, while eggshell lime contained minor impurities. Indigo cakes produced from snail shell lime had slightly lower yield (29.71% of commercial lime) but higher color intensity (+28.84%) with a less greenish-blue hue, and exhibited superior storage stability for up to 12 weeks compared to commercial indigo. Eggshell-derived indigo cakes had lower yield (38.21%) and color intensity (43.18% of commercial lime) but produced brighter, distinctly blue tones. The preparation of indigo dyeing solution from these indigo cakes required only 10 minutes before being ready for use, whereas commercial lime-based indigo required longer preparation. Cotton fabrics dyed with snail shell indigo exhibited superior colorfastness to sunlight (ΔE* = 19.12%) and washing (ΔE* = 4.38%) compared to fabrics dyed with commercial lime, while eggshell indigo produced fabrics with high color intensity (K/S = 67.41%) but lower sunlight fastness (ΔE* = 6.62%). All dyed fabrics showed blue-green hues (a* < 0, b* < 0). These results demonstrate that lime produced from household waste, especially golden apple snail shells and eggshells, can effectively replace commercial lime, reduce dye preparation time, and promote sustainable use of local resources in community-based indigo production.
GRAPHICAL ABSTRACT
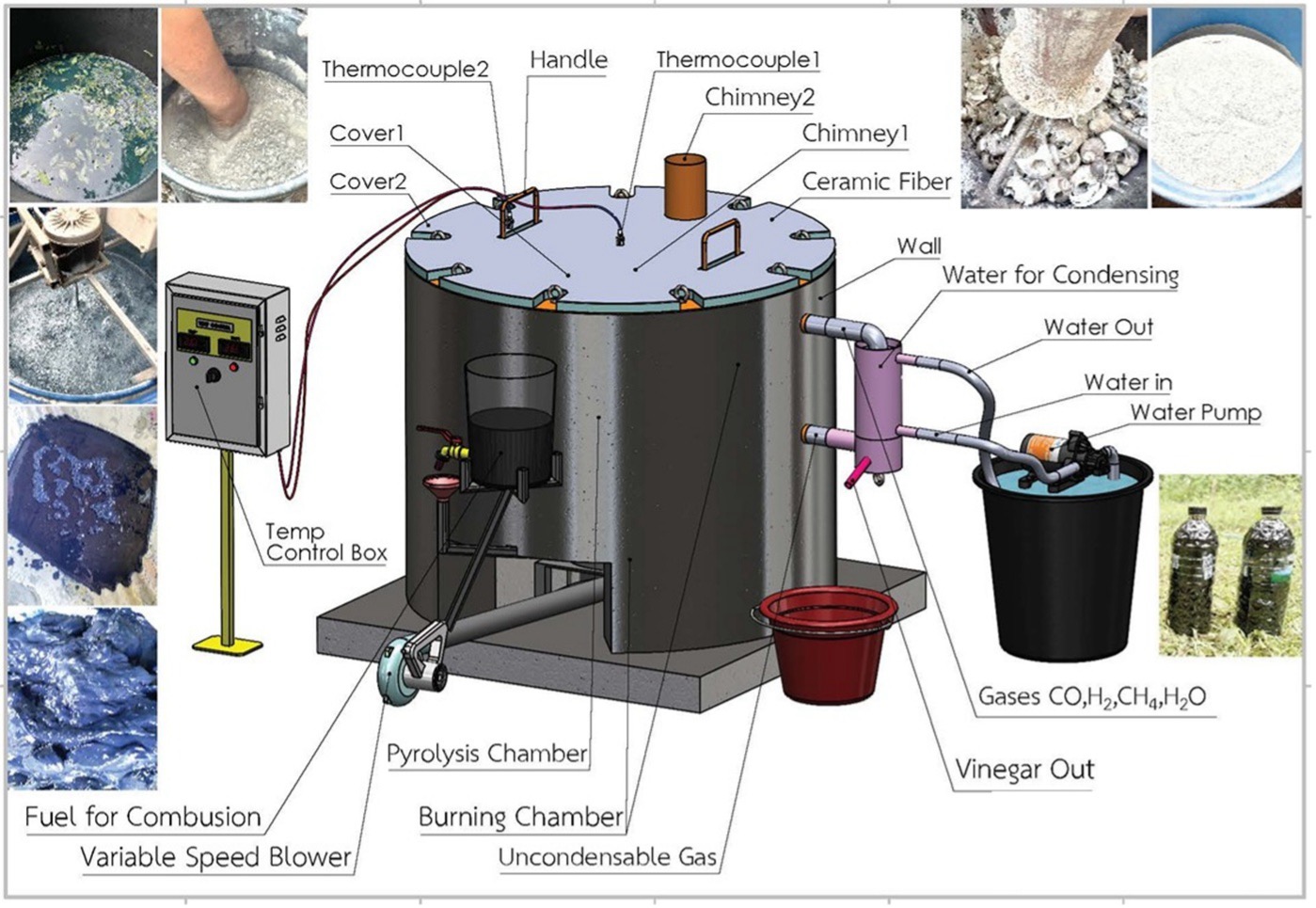
HIGHLIGHTS
- A 50-liter vertical kiln using waste vegetable oil as fuel was developed to calcine calcium-rich waste materials at 800–900 °C for 4 hours, suitable for community-scale quicklime production.
- Golden apple snail shell-derived quicklime showed high purity, fine texture, and better suitability for indigo cake production compared to eggshell-derived and commercial lime.
- Indigo cake made from snail shell lime enabled faster preparation of leuco-indigo solution and resulted in dyed fabrics with superior colorfastness to sunlight and washing.
- The approach offers a low-cost, sustainable alternative to commercial lime and promotes efficient use of household waste in local natural dyeing communities.
References
Wenner, N. (2017, December). The production of indigo dye from plants (Fibershed Report, No. 1). Fibershed.
Juorema, A., & Bechtold, T. (2017). Influence of various oxidation parameters for natural indigo dye production. Cleaner Engineering and Technology, 1, 100005. https://doi.org/10.1016/j.clet.2020.100005
Ulakpa, W. C., Adaeze, I. M., Chimezie, O. A., & Olaseinde, A. (2024). Synthesis and characterization of calcium oxide nanoparticles (CaO NPs) from snail shells using hydrothermal method. Journal of the Turkish Chemical Society Section A: Chemistry, 11(2), 825–834. https://doi.org/10.18596/jotcsa.1358231
Rajathi, K., & Sridhar, S. (2018). Synthesis and characterization of Ca(OH)₂ nanoparticles in different media. Journal of Biological and Chemical Research, 35(2), 877– 882.
Kumar, P., & Prasad, B. (2018). Production of calcium oxide from waste shell materials for environmental applications: A review. Environmental Nanotechnology, Monitoring & Management, 10, 18–26. https://doi.org/10.1016/j.enmm.2018.03.002
Özlem, C. O., Carlos, R.-N., Encarnación, R.-A., Jan, E., Dionys, V. G., & Koenraad, V. B. (2012). Phase and morphology evolution of calcium carbonate precipitated by carbonation of hydrated lime. Journal of Materials Science, 47, 6151–6165. https://doi.org/10.1007/s10853-012-6442-3
Hannes, P. (2017). Lime shaft kilns. Energy Procedia, 120, 75–95. https://doi.org/10.1016/j.egypro.2017.07.151
Nadia, N., & Zulkifli, N. (2020). Thermal decomposition of calcium carbonate in chicken eggshells via calcination. Malaysian Journal of Analytical Sciences, 26(2), 282–290. https://doi.org/10.17576/mjas-2020-2602-17
Gomez, O. M.-Vazquez, Zubieta, L. F.-Otero, Londoño, S. M.-Restrepo, & Rodriguez, M. E.-Garcia. (2024). Eggshells from agro-industrial waste for the recovery of lime, portlandite, and calcite nanoparticles through the lime cycle: A circular economic approach. Sustainable Chemistry for the Environment, 5, 100073. https://doi.org/10.1016/j.rsce.2024.100073
Reis, J. B., Pelisser, G., Levandoski, W. M. K., Ferrazzo, S. T., Mota, J. D., Silveira, A. A., & Korf, E. P. (2022). Experimental investigation of binder based on rice husk ash and eggshell lime on soil stabilization under acidic attack. Scientific Reports, 12, 7542. https://doi.org/10.1038/s41598-022-11529-6
Khachani, M., El Hamidi, A., Halim, M., & Arsalane, S. (2014). Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)₂. Journal of Materials and Environmental Science, 5(2), 615–624.
Nakamura, K., Ohtani, J., & Takahashi, M. (2020). Mechanistic insights into indigo reduction in indigo fermentation. Electrochemistry, 88(6), 639–645. https://doi.org/10.5796/electrochemistry.20-00044.

Downloads
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2025 Creative Science

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.








