Cellulolytic Lactic Acid Probiotic Bacteria from Cricket Gut: Antioxidant and α-Amylase Inhibitory Activities of Their Exopolysaccharide
DOI:
https://doi.org/10.55674/cs.v18i1.262749Keywords:
Cricket gut, Lactic acid bacteria, Antioxidant activity, α-Amylase inhibitionAbstract
This study aimed to isolate cellulolytic lactic acid bacteria (LAB) with probiotic properties from the gut of crickets and to investigate the antioxidant and α-amylase inhibitory activities of the exopolysaccharide (EPS) produced by the selected bacterial strains. Cricket samples were collected from four farms in Ubon Ratchathani Province, Thailand. A total of 14 LAB isolates were obtained and characterized based on their morphological and biochemical properties. The isolates were subsequently screened for their cellulolytic activity, and only one isolate, designated FF02041, exhibited cellulase enzyme production. Comparative analysis of the 16S rRNA gene sequence (1,282 bp) identified FF02041 as Lactobacillus sp. This isolate was further evaluated for probiotic potential and demonstrated tolerance to both acidic conditions and bile salts. The EPS extracted from isolate FF02041 exhibited DPPH and ABTS free radical scavenging activities of 15.93% and 25.59%, respectively, at a concentration of 10 mg mL-1. Additionally, the EPS showed ferric reducing antioxidant power (FRAP) of 21.74 ± 0.06 mg Fe²⁺ equivalents per gram of EPS. Furthermore, at the same concentration (10 mg mL-1), the EPS displayed α-amylase inhibitory activity with an inhibition rate of 49.61%, corresponding to 22.74 ± 0.09 mg acarbose equivalents (AE) per gram of EPS. These findings highlight the cricket gut represents a novel reservoir of functional probiotics and bioactive EPS with strong potential for nutraceutical and functional food development.
GRAPHICAL ABSTRACT
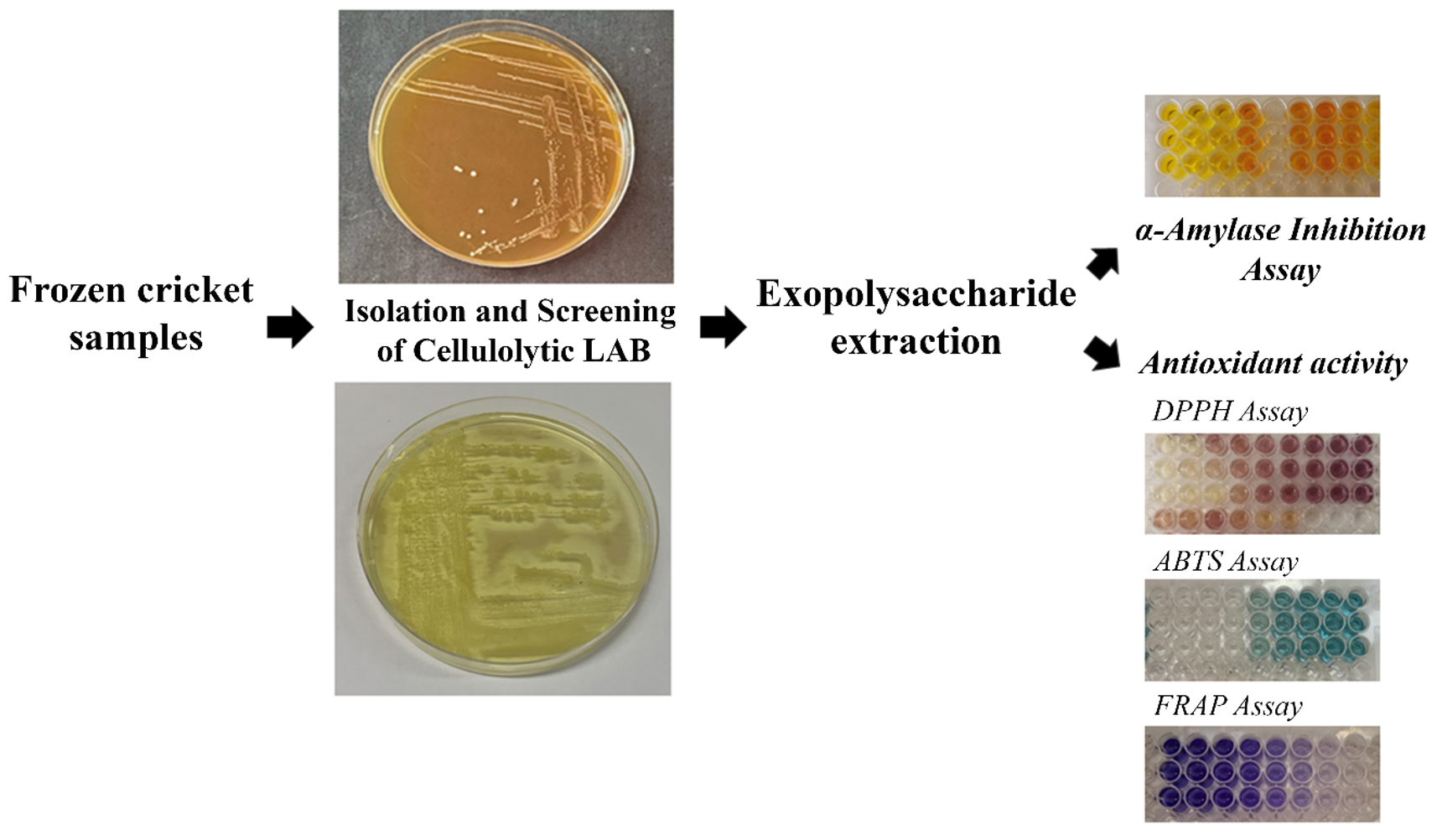
HIGHLIGHTS
- Isolation of Cellulolytic Lactic Acid Probiotic Bacteria from Cricket Gut
References
Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). (2002). Guidelines for the evaluation of probiotics in food: Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. London, Ontario.
Jang, M. H., & Kim, M. D. (2011). β‑1,4‑Xylosidase activity of Leuconostoc lactic acid bacteria isolated from kimchi. Korean Journal of Food Science and Technology, 43(2), 169–175. https://doi.org/10.9721/KJFST.2011.43.2.169
Min, K. A., & Chung, C. H. (2016). Yogurt production using exo-polysaccharide-producing Leuconostoc and Weissella isolates from kimchi. Korean Journal of Food Science and Technology, 48(3), 231–240. https://doi.org/10.9721/KJFST.2016.48.3.231
Yu, H. S., Jang, H. J., Lee, N. K., & Paik, H. D. (2019). Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi. LWT, 112, 108229. https://doi.org/10.1016/j.lwt.2019.05.127
Coniglio, M. V., Luna, M. J., Provensal, P., Watson, S., Ortiz, M. E., Ludueña, H. R., Cavaglieri, L., & Magnoli, A. P. (2023). Use of the probiotic Saccharomyces cerevisiae var. boulardii RC009 in the rearing stage of calves. International Journal of Agriculture and Biosciences, 12(3), 188–192. https://doi.org/10.47278/journal.ijab/2023.063
Park, Y. H., Hamidon, F., Rajangan, C., Soh, K. P., Gan, C. Y., Lim, T. S., Abdullah, W. N. W., & Liong, M. T. (2016). Application of probiotics for the production of safe and high-quality poultry meat. Food Science of Animal Resources, 36(5), 567–576. https://doi.org/10.5851/kosfa.2016.36.5.567
Lee, K. W., Shim, J. M., Park, S. K., Heo, H. J., Kim, H. J., Ham, K. S., & Kim, J. H. (2016). Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. LWT - Food Science and Technology, 71, 130–137. https://doi.org/10.1016/j.lwt.2016.03.029
Kechagia, M., Basoulis, D., Konstantopoulou, S., Dimitriadi, D., Gyftopoulou, K., Skarmoutsou, N., & Fakiri, E. M. (2013). Health benefits of probiotics: A review. ISRN Nutrition, 2013, 481651. https://doi.org/10.5402/2013/481651
Ojha, B. K., Singh, P. K., & Shrivastava, N. (2019). Chapter 7 - Enzymes in the animal feed industry. In M. Kuddus (Ed.), Enzymes in Food Biotechnology (pp. 93–109). Academic Press. https://doi.org/10.1016/B978-0-12-813280-7.00007-4
Srifani, A., Mirnawati, Marlida, Y., Rizal, Y., & Nurmiati. (2024). Isolation and characterization of cellulolytic lactic acid bacteria from soymilk waste as probiotic candidates for broiler. International Journal of Veterinary Science, 13(1), 108–114. https://doi.org/10.47278/journal.ijvs/2023.067
Taras, D., Vahjen, W., Macha, M., & Simon, O. (2005). Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Archives of Animal Nutrition, 59(6), 405–417. https://doi.org/10.1080/17450390500353168
Scharek, L., Guth, J., Filter, M., & Schmidt, M. F. (2007). Impact of the probiotic bacteria Enterococcus faecium NCIMB 10415 (SF68) and Bacillus cereus var. toyoi NCIMB 40112 on the development of serum IgG and faecal IgA of sows and their piglets. Archives of Animal Nutrition, 61(4), 223–234. https://doi.org/10.1080/17450390701431540
Salimi, F., & Farrokh, P. (2023). Recent advances in the biological activities of microbial exopolysaccharides. World Journal of Microbiology and Biotechnology, 39, 213. https://doi.org/10.1007/s11274-023-03660-x
Yang, Y., Jiang, G., & Tian, Y. (2023). Biological activities and applications of exopolysaccharides produced by lactic acid bacteria: A mini-review. World Journal of Microbiology and Biotechnology, 39, 155. https://doi.org/10.1007/s11274-023-03610-7
Patel, A., Prajapati, J. B., Holst, O., & Ljungh, A. (2014). Determining probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented food products. Food bioscience, 5, 27-33. https://doi.org/10.1016/j.fbio.2013.10.002
Oboh, G., Ogunsuyi, O. B., Ogunbadejo, M. D., & Adefegha, S. A. (2016). Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. Journal of Food and Drug Analysis, 24(3), 627–634. https://doi.org/10.1016/j.jfda.2016.03.003
Chiasson, J. L., Josse, R. G., Gomis, R., Hanefeld, M., Karasik, A., & Laakso, M. (2002). Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. The Lancet, 359(9323), 2072–2077. https://doi.org/10.1016/S0140-6736(02)08905-5
Ron, Y., Wainstein, J., Leibovitz, A., Monastirsky, N., Habot, B., Avni, Y., & Segal, R. (2002). The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. The Journal of Gerontology: Series A, 57(2), M111–M114. https://doi.org/10.1093/gerona/57.2.M111
Rajendiran, D., Packirisamy, S., & Gunasekaran, K. (2018). A review on role of antioxidants in diabetes. Asian Journal of Pharmaceutical and Clinical Research, 11, 48–53. https://doi.org/10.22159/ajpcr.2018.v11i2.23241
Sasikumar, K., Vaikkath, D. K., Devendra, L., & Nampoothiri, K. M. (2017). An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresource Technology, 241, 1152–1156. https://doi.org/10.1016/j.biortech.2017.05.075
Ramchandran, L., & Shah, N. P. (2009). Effect of exopolysaccharides and inulin on the proteolytic, angiotensin-I-converting enzyme-and α-glucosidase-inhibitory activities as well as on textural and rheological properties of low-fat yogurt during refrigerated storage. Journal of Dairy Science, 89, 583–600. https://doi.org/10.1051/dst/2009039
Peng, C., Sun, W., Dong, X., Zhao, L., & Hao, J. (2021). Isolation, identification and utilization of lactic acid bacteria from silage in a warm and humid climate area. Scientific Reports, 11, 12586. https://doi.org/10.1038/s41598-021-92034-0
Rhaiem, N., Chahboun, N., Inekach, S., & Ouhssine, M. (2016). Identification and characterization of lactic acid bacteria isolated from cow milk and olives brine. Journal of Materials and Environmental Science, 7(5), 1504–1509.
Mandel, M., & Weber, J. (1969). The Production of Cellulases. Advances in Chemistry, 95, 391–413.
Frank, J. A., Reich, C. I., Sharma, S., Weisbaum, J. S., Wilson, B. A., & Olsen, G. J. (2008).
Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Applied and Environmental Microbiology, 74(8), 2461–2470. https://doi.org/10.1128/AEM.02272-07
Sanger, F., Nicklen, S., & Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 74(12), 5463–5467. https://doi.org/10.1073/pnas.74.12.5463
[Torkian, B., Hann, S., Preisner, E., & Norman, R. S. (2020). BLAST-QC: Automated analysis of BLAST results. Environmental Microbiome, 15, 15. https://doi.org/10.1186/s40793-020-00361-y
Tamura, K., Stecher, G., & Kumar, S. (2021). MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38(7), 3022–3027. https://doi.org/10.1093/molbev/msab120
Kimura, M. (1980). A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16(2), 111–120. https://doi.org/10.1007/BF01731581
Liong, M.T., & Shah, N.P. (2005). Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. Journal of Dairy Science, 88(1), 55–66. https://doi.org/10.3168/jds.S0022-0302(05)72662-X
Hu, P.L., Yuan, Y.H., Yue, T. & Guo, C.F. (2018). A new method for the in vitro determination of the bile tolerance of potentially probiotic lactobacilli. Applied Microbiology and Biotechnology, 102(2), 1903–1910. https://doi.org/10.1007/s00253-018-8742-x
Sharma, K., Sharma, N., Handa, S., & Pathania, S. (2020). Purification and characterization of novel exopolysaccharides produced from Lactobacillus paraplantarum KM1 isolated from human milk and its cytotoxicity. Journal of Genetic Engineering and Biotechnology, 18(1), 56. https://doi.org/10.1186/s43141-020-00063-5
Saeting, O., Chandarajoti, K., Phongphisutthinan, A., Hongsprabhas, P., & Sae-Tan, S. (2021). Water extract of mungbean (Vigna radiata L.) inhibits protein tyrosine phosphatase-1B in insulin-resistant HepG2 cells. Molecules, 26(5), 1452. https://doi.org/10.3390/molecules26051452
Xiang, Y., Haixia, W., Lijuan, M., & Yanduo, T. (2018). Isolation, identification of purification antioxidants and from Lepidium latifolium extracts. Medicinal Chemistry Research, 27, 37–45. https://doi.org/10.1007/s00044-017-2042-3
Kim, E. K., Lee, S. J., Lim, B. O., Jeon, Y. J., Song, M. D., Park, T. K., Lee, K. H., Kim, B. K., Lee, S. R., Moon, S. H., Jeon, B. T., & Park, P. J. (2008). Antioxidative and neuroprotective effects of enzymatic extracts from leaves of Perilla frutescens var. japonica. Food Science and Biotechnology, 17(2), 279–286.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
Thanananta, N., Ketsawasdiwong, N., & Thanananta, T. (2016). Screening of Lactic Acid Bacteria from Thai’s Chilli Pastes. Thai Journal of Science and Technology, 5(1), 67–76. https://doi.org/10.14456/tjst.2016.1
Saratale, G. D., Saratale, R. G., & Oh, S. E. (2012). Production and characterization of multiple cellulolytic enzymes by isolated Streptomyces sp. MDS. Biomass and Bioenergy, 47, 302–315. https://doi.org/10.1016/j.biombioe.2012.09.030
Liao, Y., Wu, S., Zhou, G., Mei, S., Yang, Z., Li, S., Jin, Z., Deng, Y., Wen, M., & Yang, Y. (2024). Cellulolytic Bacillus cereus produces a variety of short-chain fatty acids and has potential as a probiotic. Microbiology Spectrum, 12(4), e0326723. https://doi.org/10.1128/spectrum.03267-23
Lynd, L. R., Paul, J. W., van Zyl, W. H., & Pretorius, I. S. (2002). Microbial cellulose Utilization: Fundamentals and biotechnology. Microbiology and Molecular Biology Reviews, 66(3), 506–577. https://doi.org/10.1128/MMBR.66.3.506-577.2002
Ghazanfar, M., Irfan, M., Nadeem, M., Shakir, H. A., Khan, M., Ali, S., Saeed, S., & Mehmood, T. (2021). Isolation of cellulolytic bacteria from soil and valorization of different lignocellulosic wastes for cellulase production by submerged fermentation. Cellulose Chemistry and Technology, 55(7-8), 821–828. https://doi.org/10.35812/CelluloseChemTechnol.2021.55.69
Chun, J., Oren, A., Ventosa, A., Christensen, H., Arahal, D. R., da Costa, M. S., Rooney, A. P., Yi, H., Xu, X. W., De Meyer, S., & Trujillo, M. E. (2018). Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. International Journal of Systematic and Evolutionary Microbiology, 68(1), 461–466. https://doi.org/10.1099/ijsem.0.002516
Hassanzadazar, H., Ehsani, A., Mardani, K., & Hesari, J. (2012). Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Veterinary Research Forum, 3(3), 181–185.
Soltan Dallal, M. M., Zamaniahari, S., Davoodabadi, A., Hosseini, M., & Rajabi, Z. (2017). Identification and characterization of probiotic lactic acid bacteria isolated from traditional Persian pickled vegetables. GMS Hygiene and Infection Control, 12, 15. https://doi.org/10.3205/dgkh000300
Thaweesang, S., & Leenanon, B. (2016). Survival of Lactobacillus acidophilus TISTR1338 in bile salt stress conditions. Asian Journal of Microbiology, Biotechnology and Environmental Sciences, 18(3), 569–579.
Safitri, R., Khotimah, K., Balia, R., Saputra, M. I., Miranti, M., & Utama, G. L. (2016). The tolerance of Lactobacillus paracasei and Lactobacillus curvatus originated from bovine colostrum towards acidity and bile salts as a probiotics candidate. Advance Journal of Food Science and Technology, 11(1), 60–63. https://doi.org/10.19026/AJFST.11.2355
Karasov, W. H., & Douglas, A. E. (2013). Comparative digestive physiology. Comprehensive Physiology, 3(2), 741–783. https://doi.org/10.1002/j.2040-4603.2013.tb00501.x
Delamotte, P. & Montagne, J. (2024). Dietary Lipids and Their Metabolism in the Midgut. In N. Rezaei, O. Steinlein, J. Xiao, A. Rosenhouse-Dantsker & R. Gerlai (Eds), Advances in Experimental Medicine and Biology (pp. 1-32). Springer, Cham. https://doi.org/10.1007/5584_2024_835
Ullah, F. S., Saeed, M., Shabbir, M. A., & Israr, B. (2025). Microencapsulation of Lactobacillus acidophilus LA 832 and Bifidobacterium longum BL 101 by using sodium alginate and chitosan to improve the survival in simulated gastrointestinal conditions. Journal of Food Science and Technology. https://doi.org/10.1007/s13197-025-06345-5
Li, S., Huang, R., Shah, N. P., Tao, X., Xiong, Y., & Wei, H. (2014). Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. Journal of Dairy Science, 97(12), 7334–7343. https://doi.org/10.3168/jds.2014-7912
Imran, M. Y. M., Reehana, N., Jayaraj, K. A., Ahamed, A. A. P., Dhanasekaran, D., Thajuddin, N., Alharbi, N. S., & Muralitharan, G. (2016). Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. International Journal of Biological Macromolecules, 93, 731–745. https://doi.org/10.1016/j.ijbiomac.2016.09.007
Bouzaiene, T., Mohamedhen Vall, M., Ziadi, M., Ben Rejeb, I., Yangui, I., Aydi, A., Ouzari, I., & Moktar, H. (2024). Exopolysaccharides from Lactiplantibacillus plantarum C7 exhibited antibacterial, antioxidant, anti-enzymatic, and prebiotic activities. Fermentation, 10(7), 339. https://doi.org/10.3390/fermentation10070339
Dilna, S. V., Surya, H., Aswathy, R. G., Varsha, K. K., Sakthikumar, D. N., Pandey, A., & Nampoothiri, K. M. (2015). Characterization of an exopolysaccharide with potential health benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT - Food Science and Technology, 64(2), 1179–1186. https://doi.org/10.1016/j.lwt.2015.07.040
Sasikumar, K., Vaikkath, D. K., Devendra, L., & Nampoothiri, K. M. (2017). An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional food. Bioresource Technology, 241, 1152–1156. https://doi.org/10.1016/j.biortech.2017.05.075
Wang, J., Zhang, J., Guo, H., Cheng, Q., Abbas, Z., Tong, Y., Yang, T., Zhou, Y., Zhang, H., Wei, X., Si, D., & Zhang, R. (2023). Optimization of exopolysaccharide produced by Lactobacillus plantarum R301 and its antioxidant and anti-inflammatory activities. Foods, 12(13), 2481. https://doi.org/10.3390/foods12132481
Adebayo-Tayo, B., & Fashogbon, R. (2020). In vitro antioxidant, antibacterial, in vivo immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild type and mutant Lactobacillus delbrueckii subsp. bulgaricus. Heliyon, 6(2), e03268. https://doi.org/10.1016/j.heliyon.2020.e03268
Fernandes, P. A. R., & Coimbra, M. A. (2023). The antioxidant activity of polysaccharides: A structure–function relationship overview. Carbohydrate Polymers, 314, 120965. https://doi.org/10.1016/j.carbpol.2023.120965
Chen, N., Jiang, T., Xu, J., Xi, W., Shang, E., Xiao, P., & Duan, J. A. (2024). The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. International Journal of Biological Macromolecules, 270(Part 2), 132391. https://doi.org/10.1016/j.ijbiomac.2024.132391
He, P., Zhang, A., Zhang, F., Linhardt, R. J., & Sun, P. (2016). Structure and bioactivity of a polysaccharide containing uronic acid from Polyporus umbellatus sclerotia. Carbohydrate Polymers, 152, 222–230. https://doi.org/10.1016/j.carbpol.2016.07.010
Yin, L., Fu, S., Wu, R., Wei, S., Yi, J., Zhang, L. M., & Yang, L. (2020). Chain conformation of an acidic polysaccharide from green tea and related mechanism of α-amylase inhibitory activity. International Journal of Biological Macromolecules, 164, 1124–1132. https://doi.org/10.1016/j.ijbiomac.2020.07.125

Downloads
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2025 Creative Science

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.








