Utilization of Alum Sludge from Water Treatment Plant as an Adsorbent for Hydrogen Sulfide Removal
Keywords:
Adsorption, Alum sludge, Hydrogen sulfide, Water treatment plant sludgeAbstract
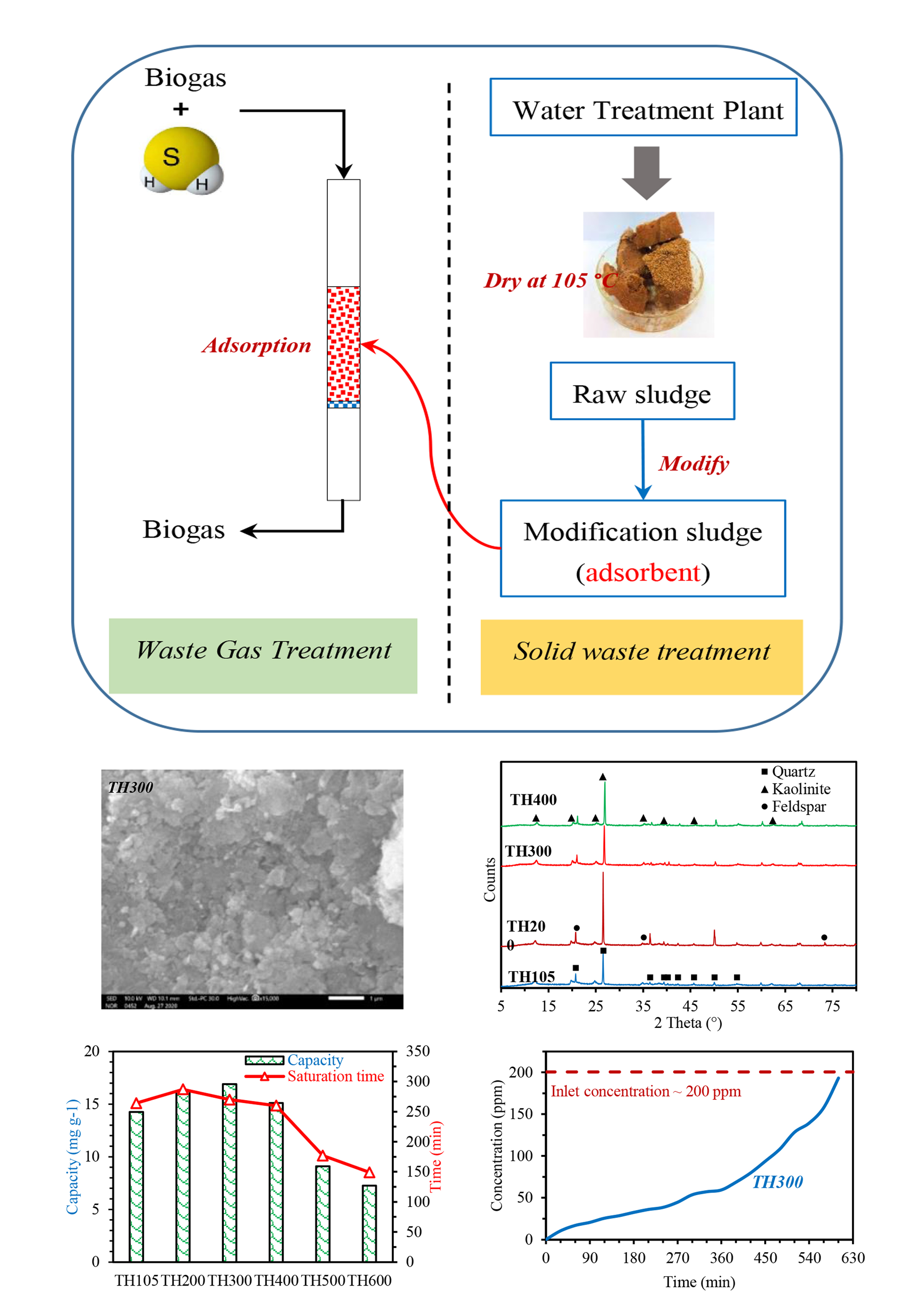
Utilizing waste is one of the research directions that receive a lot of attention, especially in the field of the environment. In this study, the H2S adsorption capacity of modified sludge from Tan Hiep Water Treatment Plant (Ho Chi Minh City, Vietnam) was investigated. Scanning electron microscopy, energy- dispersive X-ray spectroscopy, and X-ray diffraction were used to analyze material properties. The effects of operating conditions such as H2S concentration and airflow rate were also surveyed. The analysis show the Tan Hiep Water Treatment Plant sludge contains metallic elements that are mainly aluminum. The appropriate temperature for calcining the raw sludge was 300 °C, and the Langmuir isotherm equation is suitable to describe H2S adsorption process in the concentration range of 50 – 400 ppm with R2 = 0.99. Under the optimal operating conditions (e.g., H2S concentration of 200 ppm and flowrate of 1 L min–1), the adsorption capacity of the alum sludge heated at 300 °C reached 36.65 mgH2S g–1, which is potential for practical H2S adsorption in biogas.
References
[1] L.B. Allegue, J. Hinge, Biogas upgrading evaluation of methods for H2S removal, Danish Technological Institute 31 (2014).
[2] V. Garcia-Arriaga, J. Alvarez-Ramirez, M. Amaya, E. Sosa, H2S and O2 influence on the corrosion of carbon steel immersed in a solution containing 3 M diethanolamine, Corros Sci 52 (2010) 2268-79.
[3] S. Zhang, M. Osterman, A. Shrivastava, R. Kang, M.G. Pecht, The Influence of H2S Exposure on Immersion-Silver-Finished PCBs Under Mixed-Flow Gas Testing, IEEE Transactions on Device and Materials Reliability 10 (2009) 71-81.
[4] J. Zhang, Z. Tong, Study on catalytic wet oxidation of H2S into sulfur on Fe/Cu catalyst, Journal of Natural Gas Chemistry 15 (2006) 63-9.
[5] C. Petit, B. Mendoza, T.J. Bandosz, Hydrogen sulfide adsorption on MOFs and MOF/graphite oxide composites, ChemPhysChem 11 (2010) 3678-84.
[6] M. Ozekmekci, G. Salkic, M.F. Fellah, Use of zeolites for the removal of H2S: A mini-review, Fuel Process Technol 139 (2015) 49-60.
[7] A.G. Georgiadis, N.D. Charisiou, M.A. Goula, Removal of hydrogen sulfide from various industrial gases: A review of the most promising adsorbing materials, Catalysts 10 (2020) 521.
[8] N.N. Huy, V.T.T. Thuy, N.H. Thang, N.T. Thuy, T.T. Khoi, D. Van Thanh, Facile one-step synthesis of zinc oxide nanoparticles by ultrasonic-assisted precipitation method and its application for H2S adsorption in air, J Phys Chem Solids 132 (2019) 99-103.
[9] L.P.T. Hien, P.D. Thanh, B.K. Le, D.Q. Vinh, T.D. Vi, P.H. Hai, V.T.T. Thuy, N.N. Huy, Preparation of activated red mud and its application for removal of hydrogen sulfide in air, Science & Technology Development Journal-Engineering and Technology 2 (2019) SI40-SI5.
[10] H. Wu, Y. Zhu, S. Bian, J.H. Ko, S.F.Y. Li, Q. Xu, H2S adsorption by municipal solid waste incineration (MSWI) fly ash with heavy metals immobilization, Chemosphere 195 (2018) 40-7.
[11] F.G. Ortiz, P. Aguilera, P. Ollero, Biogas desulfurization by adsorption on thermally treated sewage-sludge, Sep Purif Technol 123 (2014) 200-13.
[12] A. Sales, F.R. de Souza, F.d.C.R. Almeida, Mechanical properties of concrete produced with a composite of water treatment sludge and sawdust, Construction and Building Materials 25 (2011) 2793-8.
[13] C. Huang, J.R. Pan, Y. Liu, Mixing water treatment residual with excavation waste soil in brick and artificial aggregate making, J Environ Eng 131 (2005) 272-7.
[14] K. Dassanayake, G. Jayasinghe, A. Surapaneni, C. Hetherington, A review on alum sludge reuse with special reference to agricultural applications and future challenges, Waste Manage 38 (2015) 321-35.
[15] K.C. Makris, W.G. Harris, G.A. O'Conno, T.A. Obreza, Phosphorus immobilization in micropores of drinking-water treatment residuals: implications for long-term stability, Environ Sci Technol 38 (2004) 6590-6.
[16] M. Razali, Y. Zhao, M. Bruen, Effectiveness of a drinking-water treatment sludge in removing different phosphorus species from aqueous solution, Sep Purif Technol 55 (2007) 300-6.
[17] Y.W. Chiang, K. Ghyselbrecht, R.M. Santos, J.A. Martens, R. Swennen, V. Cappuyns, B. Meesschaert, Adsorption of multi-heavy metals onto water treatment residuals: sorption capacities and applications, Chem Eng J 200 (2012) 405-15.
[18] Y.-F. Zhou, R.J. Haynes, Removal of Pb (II), Cr (III) and Cr (VI) from aqueous solutions using alum-derived water treatment sludge, Water, Air, Soil Pollut 215 (2011) 631-43.
[19] A.G. Caporale, P. Punamiya, M. Pigna, A. Violante, D. Sarkar, Effect of particle size of drinking-water treatment residuals on the sorption of arsenic in the presence of competing ions, J Hazard Mater 260 (2013) 644-51.
[20] S. Polruang, P. Banjerdkij, S. Sirivittayapakorn, Use of drinking water sludge as adsorbent for H2S gas removal from biogas, Environment Asia 10 (2017) 73-80.
[21] W.L. McCabe, J.C. Smith, P. Harriott. Unit operations of chemical engineering1985.
[22] H.M. Owaid, R. Hamid, M. Taha, Influence of thermally activated alum sludge ash on the engineering properties of multiple-blended binders concretes, Construction and Building Materials 61 (2014) 216-29.
[23] H. Awab, P.T. Paramalinggam, A.R.M. Yusoff, Characterization of alum sludge for reuse and disposal, Malaysian Journal of Fundamental and Applied Sciences 8 (2012).
[24] S. Monteiro, J. Alexandre, J. Margem, R. Sánchez, C. Vieira, Incorporation of sludge waste from water treatment plant into red ceramic, Construction and Building Materials 22 (2008) 1281-7.
[25] T.J. Bandosz, On the adsorption/oxidation of hydrogen sulfide on activated carbons at ambient temperatures, J Colloid Interface Sci 246 (2002) 1-20.
[26] A. Ros, M.A. Montes-Moran, E. Fuente, D.M. Nevskaia, M.J. Martin, Dried sludges and sludge-based chars for H2S removal at low temperature: influence of sewage sludge characteristics, Environ Sci Technol 40 (2006) 302-9.
[27] X. Xu, X. Cao, L. Zhao, T. Sun, Comparison of sewage sludge-and pig manure-derived biochars for hydrogen sulfide removal, Chemosphere 111 (2014) 296-303.