Green extraction of anthocyanins from roselle: A comparative evaluation of extraction techniques and solvents
Main Article Content
Abstract
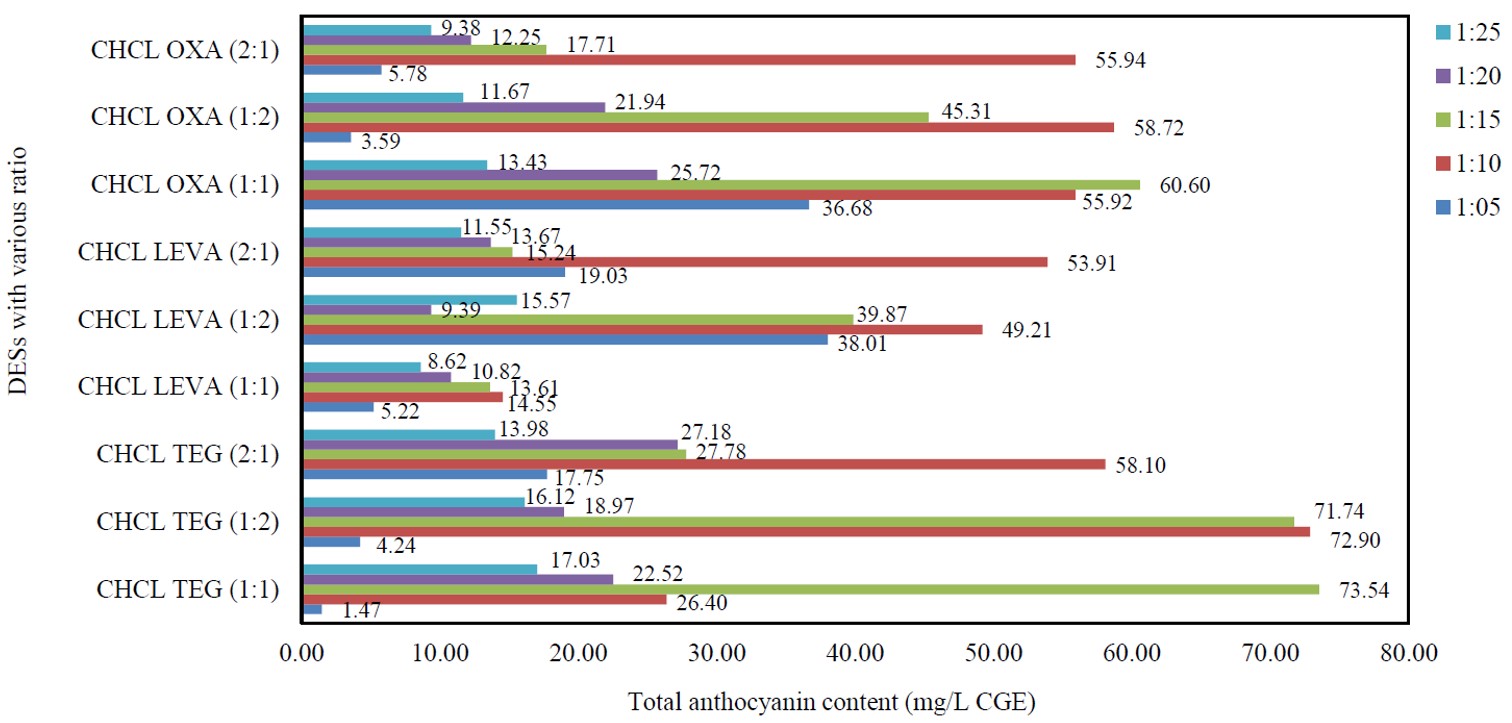
Due to the abundance of roselle in nature, the potential of the roselle to be a source of natural colorant with high anthocyanin content was explored. Moreover, in conjunction with the 12 principles and practices of Green Chemistry, one of the promising solvents that can offer greenness is deep eutectics solvents (DESs) with broad tunability and high selectivity as an alternative to volatile organic solvents. DESs are solvents comprising a combination of hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD) with lower melting points than their parents’ salts. Therefore, this study explored the potential of DESs as an additive to water as solvents for the extraction of total anthocyanins content (TAC) from roselle extracts. By optimizing extraction parameters, including the best DESs, solid-to-solvent ratio, particle size, and extraction method, this study identified the most effective conditions. Based on the results obtained, the best solvents were choline chloride and triethylene glycol at a ratio of (1:1). Meanwhile, the best solid-to-solvent ratio is 1:15 (g/g). Furthermore, this study obtained the best extraction condition at 750 µm of average particle size using ultrasonic-assisted extraction (UAE) with a yield of 119.02 mg/L cyanidin-3-glucoside equivalence (GCE). As a result, UAE is a promising way to get anthocyanins out of roselle, using DESs as an extra solvent and following the set experimental rules. This study highlights the potential of DESs and the UAE to recover valuable compounds from natural sources and promote sustainable and environmentally friendly practices.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Aminul Islam AKM, Jamini TS, Mominul Islam AKM, Yeasmin S. Roselle: A functional food with high nutritional and medicinal values. Fund Appl Agric. 2016;1(2):44-9.
Shruthi VH, Ramachandra CT, Nidoni U, Hiregoudar S, Naik N, Kurubar AR. Roselle (Hibiscus Sabdariffa L.) as a source of natural color: a review. Plant Arch. 2016;16(2):515-22.
Riaz G, Naik SN, Garg M, Chopra R. Phytochemical composition of an underutilized plant sorrel/roselle (Hibiscus Sabdariffa L.) cultivated in India. Lett Appl NanoBioScience. 2021;10(2):2138-47.
Chin KL, Zhen J, Qi Y, Chin SL, Breithaupt M, Wu QL, et al. A comparative evaluation: Phytochemical composition and antioxidant capacity of three roselle (Hibiscus sabdariffa L.) accessions. Acta Hortic. 2016;1125:99-108.
Pozos GIP, Ruiz-López MA, Nátera JFZ, Moya CÁ, Ramírez LB, Silva MR, et al. Antioxidant capacity and antigenotoxic effect of Hibiscus sabdariffa L. extracts obtained with ultrasound-assisted extraction process. Appl Sci. 2020;10(2):560.
Riaz G, Chopra R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmacother. 2018;102:575-86.
Abubakar AR, Haque M. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci. 2020;12(1):1-10.
Gałuszka A, Migaszewski Z, Namieśnik J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal Chem. 2013;50:78-84.
Dean JR. Microwave extraction. In: Pawliszyn J, editor. Comprehensive sampling and sample preparation volume 2. Amsterdam: Elsevier; 2012. p. 135-49.
Lavilla I, Bendicho C. Fundamentals of ultrasound-assisted extraction. In: González HD, Muñoz MJG, editors. Water extraction of bioactive compounds. Amsterdam: Elsevier; 2017. p. 291-316.
Joshi DR, Adhikari N. An overview on common organic solvents and their toxicity. J Pharm Res Int. 2019;28(3):1-18.
Taghavi T, Patel H, Rafie R. Comparing pH differential and methanol-based methods for anthocyanin assessments of strawberries. Food Sci Nutr. 2021;10(7):2123-31.
Warner JC, Cannon AS, Dye KM. Green chemistry. Environ Impact Assess Rev. 2004;24(7-8):775-99.
Smith EL, Abbott AP, Ryder KS. Deep Eutectic Solvents (DESs) and their applications. Chem Rev. 2014;114(21):11060-82.
Abbott AP, Barron JC, Ryder KS, Wilson D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem - Eur J. 2007;13(22):6495-501.
Zannou O, Pashazadeh H, Ibrahim SA, Koca I, Galanakis CM. Green and highly extraction of phenolic compounds and antioxidant capacity from kinkeliba (Combretum micranthum G. Don) by natural deep eutectic solvents (NADESs) using maceration, ultrasound-assisted extraction and homogenate-assisted extraction. Arab J Chem. 2022;15(5):103752.
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003;(1):70-1.
Dai Y, Witkamp GJ, Verpoorte R, Choi YH. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015;187:14-9.
Dai Y, Rozema E, Verpoorte R, Choi YH. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J Chromatogr A. 2016;1434:50-6.
Roy D, Miller L. Exploring the utility of natural deep eutectic solvents as additives in super/subcritical fluid chromatography- insights into chiral recognition mechanism. Anal Chim Acta. 2022;1200:339584.
Omar KA, Sadeghi R. Database of deep eutectic solvents and their physical properties: a review. J Mol Liq. 2023;384:121899.
Tan PW, Tan CP, Ho CW. Antioxidant properties: effects of solid-to-solvent ratio on antioxidant compounds and capacities of Pegaga (Centella asiatica). Int Food Res J. 2011;18:557-62.
Azmi SN, Ruslan MSH. Extraction of essential oil from kaffir lime (Citrus hystrix) leaves using microwave-assisted extraction. Malaysian J Chem Eng Technol. 2022;5(2):84-93.
Minitab Inc. What are response surface designs, central composite designs, and Box-Behnken designs?, Response Surface Design [Internet]. 2010 [cited 2022 Jan 27]. Available from: https://support.minitab.com/en-us/minitab/18/help-and-how-to/modeling-statistics/doe/supporting-topics/response-surface-designs/response-surface-central-composite-and-box-behnken-designs/.
Alañón ME, Ivanović M, Pimentel-Mora S, Borrás-Linares I, Arráez-Román D, Segura-Carretero A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res Int. 2020;137:109646.
Amaya-Cruz DM, Perez-Ramirez IF, Ortega-Diaz D, Rodriguez-Garcia ME, Reynoso-Camacho R. Roselle (Hibiscus sabdariffa) by-product as functional ingredient: effect of thermal processing and particle size reduction on bioactive constituents and functional, morphological, and structural properties. J Food Meas Charact. 2018;12:135-44.
Aryanti N, Nafiunisa A, Wardhani DH. Conventional and ultrasound-assisted extraction of anthocyanin from red and purple roselle (Hibiscus sabdariffa L.) calyces and characterization of its anthocyanin powder. Int Food Res J. 2019;26(2):529-35.
Aneke NN, Okonkwo WI, Ezeoha SL, Okafor GI, Anyanwu CN. Optimization of anthocyanin extraction from roselle (Hibiscus sabdariffa) calyces: RSM, kinetic modelling, mass transfer and thermodynamic studies. J Res Innov Food Sci Technol. 2023;11(4):437-50.
Selim KA, Khalil KE, Abdel-Bary MS, Abdel-Azeim NA. Extraction, encapsulation and utilization of red pigments from roselle (Hibiscus sabdariffa L.) as natural food colourants. Alex J Food Sci Technol. 2008;5(2):7-20.
Redzuan S, Ho CY, Idham Z, Yusuf S, Putra NR, Yunus MAC, et al. Optimization of anthocyanins extracts from roselle (Hibiscus sabdarifa) petals using ultrasonic-assisted extraction method. In: Zaini MAA, Jusoh M, Othman N, editors. Proceedings of the 3rd International Conference on Separation Technology. Lecture Notes in Mechanical Engineering. Singapore: Springer; 2021. p. 295-309.
Cid-Ortega S, Guerrero-Beltran JA. Roselle calyces particle size effect on the physicochemical and phytochemicals characteristics. J Food Res. 2014;3(5):83-94.
Roslan R, Kurnia KA, Hasanudin N, Hilmy NIMF, Ruslan MSH. Screen and design of deep eutectic solvents (DESs) for the extraction of delphinidin-3-sambubioside from Hibiscus sabdariffa via COSMO-RS. AIP Conf Proc. 2024;3041(1):050003.
Benvenutti L, Zielinski AAF, Ferreira SRS. Which is the best food emerging solvent: IL, DES or NADES?. Trends Food Sci Technol. 2019;90:133-46.
Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88(5):1269-78.
Anderson MJ, Whitcomb PJ. DOE simplified - practical tools for effective experimentation. 3rd ed. Boca Raton: CRC Press; 2015.
Pollini L, Tringaniello C, Ianni F, Blasi F, Manes J, Cossignani L. Impact of ultrasound extraction parameters on the antioxidant properties of Moringa oleifera leaves. Antioxidants. 2020;9(4):277.
Pan X, Xu L, Meng J, Chang M, Cheng Y, Geng X, et al. Ultrasound-Assisted deep eutectic solvents extraction of polysaccharides from Morchella importuna: optimization, physicochemical properties, and bioactivities. Front Nutr. 2022;9:1-11.
Kisanthia R, Hunt AJ, Sherwood J, Somsakeesit LO, Phaosiri C. Impact of conventional and sustainable solvents on the yield, selectivity, and recovery of curcuminoids from turmeric. ACS Sustain Chem Eng. 2022;10(1):104-14.
Wang J, Jing W, Tian H, Liu M, Yan H, Bi W, et al. Investigation of deep eutectic solvent-based microwave-assisted extraction and efficient recovery of natural products. ACS Sustain Chem Eng. 2020;8(32):12080-8.
Izirwan I, Munusamy TD, Hamidi NH, Sulaiman SZ. Optimization of microwave-assisted extraction of anthocyanin from Clitoria ternatea flowers. Int J Mech Eng Robot Res. 2020;9(9):1246-52.
Setyaningsih W, Putro AW, Fathimah RN, Kurnia KA, Darmawan N, Yulianto B, et al. A microwave-based extraction method for the determination of sugar and polyols: application to the characterization of regular and peaberry coffees. Arab J Chem. 2022;15(3):103660.
Yadav A, Trivedi S, Rai R, Pandey S. Densities and dynamic viscosities of (choline chloride+glycerol) deep eutectic solvent and its aqueous mixtures in the temperature range (283.15-363.15)K. Fluid Phase Equilib. 2014;367:135-42.
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. Deep eutectic Solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc. 2004;126(29):9142-7.
Abbott AP, Ahmed EI, Prasad K, Qader IB, Ryder KS. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017;448:2-8.
Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH. Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta. 2013;766:61-8.
Rashid Z, Wilfred CD, Gnanasundaram N, Arunagiri A, Murugesan T. Screening of ionic liquids as green oilfield solvents for the potential removal of asphaltene from simulated oil: COSMO-RS model approach. J Mol Liq. 2018;255:492-503.
Zhao BY, Xu P, Yang FX, Wu H, Zong MH, Lou WY. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain Chem Eng. 2015;3(11):2746-55.
Salazar-González C, Vergara-Balderas FT, Ortega-Regules AE, Guerrero-Beltrán JÁ. Antioxidant properties and color of Hibiscus sabdariffa extracts. Cien Inv Agr. 2012;39(1):79-90.
Aissaoui T, Benguerba Y, AlNashef IM. Theoretical investigation on the microstructure of triethylene glycol based deep eutectic solvents: COSMO-RS and TURBOMOLE prediction. J Mol Struct. 2017;1141:451-6.
Hayyan M, Aissaoui T, Hashim MA, AlSaadi MAH, Hayyan A. Triethylene glycol based deep eutectic solvents and their physical properties. J Taiwan Inst Chem Eng. 2015;50:24-30.
Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Med. 2018;13:1-26.
Tchabo W, Ma Y, Kwaw E, Xiao L, Wu M, Apaliya MT. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int J Food Prop. 2018;21(1):717-32.
Richardson JF, Harker JH, Backhurst JR. Chapter 10 - Leaching. Chemical engineering volume 2. Amsterdam: Elsevier; 2002. p. 502-41.
Chanioti S, Liadakis G, Tzia C. Solid–Liquid extraction. In: Varzakas T, Tzia C, editors. Food Engineering Handbook. Boca Raton: CRC Press; 2014. p. 253-86.
Hu J, Chen Y, Ni D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT - Food Sci Technol. 2012;45(1):8-12.
Amyotte PR, Eckhoff RK. Dust explosion causation, prevention and mitigation: an overview. J Chem Health Saf. 2010;17(1):15-28.
Hanula M, Wyrwisz J, Moczkowska M, Horbańczuk OK, Pogorzelska-Nowicka E, Wierzbicka A. Optimization of microwave and ultrasound extraction methods of açai berries in terms of highest content of phenolic compounds and antioxidant activity. Appl Sci. 2020;10(23):8325.
Şahin S, Pekel AG, Toprakçı İ. Sonication-assisted extraction of Hibiscus sabdariffa for the polyphenol’s recovery: application of a specially designed deep eutectic solvent. Biomass Conv Bioref. 2022;12:4959-69.
Chaves JO, de Souza MC, da Silva LC, Lachos-Perez D, Torres-Mayanga PC, da Fonseca Machado AP, et al. Extraction of flavonoids from natural sources using modern techniques. Front Chem. 2020;8:1-25.
Fitri N, Halimatussa’diah, Fitriastuti D. Comparison between maceration and microwave extraction techniques of strawberry fruit (fragaria sp) and antioxidant activity test. IOP Conf Ser: Mater Sci Eng. 2019;523:012024.
Cvjetko Bubalo M, Ćurko N, Tomašević M, Kovačević Ganić K, Radojcic Redovnikovic I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016;200:159-66.
Pinela J, Prieto MA, Pereira E, Jabeur I, Barreiro MF, Barros L, et al. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019;275:309-21.
Oliveira G, Marques C, de Oliveira A, de Almeida dos Santos A, do Amaral W, Ineu RP, et al. Extraction of bioactive compounds from Curcuma longa L. using deep eutectic solvents: In vitro and in vivo biological activities. Innov Food Sci Emerg Technol. 2021;70:102697.
Kate A, Singh A, Shahi N, Pandey JP, Prakash O. Modeling and kinetics of microwave assisted leaching based oil extraction from Bhat. J Food Process Eng. 2020;43(10):e13503.
Xiaokang W, Lyng JG, Brunton NP, Cody L, Jacquier JC, Harrison SM, et al. Monitoring the effect of different microwave extraction parameters on the recovery of polyphenols from shiitake mushrooms: comparison with hot-water and organic-solvent extractions. Biotechnol Rep. 2020;27:e00504.
Alara OR, Abdurahman NH. Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: kinetic modelling and process intensification. Ind Crops Prod. 2019;137:528-35.
Alara OR, Abdurahman NH, Ukaegbu CI, Kabbashi NA. Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing soxhlet and microwave-assisted extraction techniques. J Taibah Univ Sci. 2019;13(1):414-22.
Olalere OA, Abdurahman HN, Yunus R bin M, Alara OR, Ahmad MM, Zaki YH, et al. Parameter study, antioxidant activities, morphological and functional characteristics in microwave extraction of medicinal oleoresins from black and white pepper. J Taibah Univ Sci. 2018;12(6):730-7.
Dias ALB, Arroio Sergio CS, Santos P, Barbero GF, Rezende CA, Martínez J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum). Ultrason Sonochem. 2016;31:284-94.
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540-60.