Alstonia Scholaris Fruit Biomass, a Novel Adsorbent for the Removal of Lead from Aqueous Solution
Main Article Content
Abstract
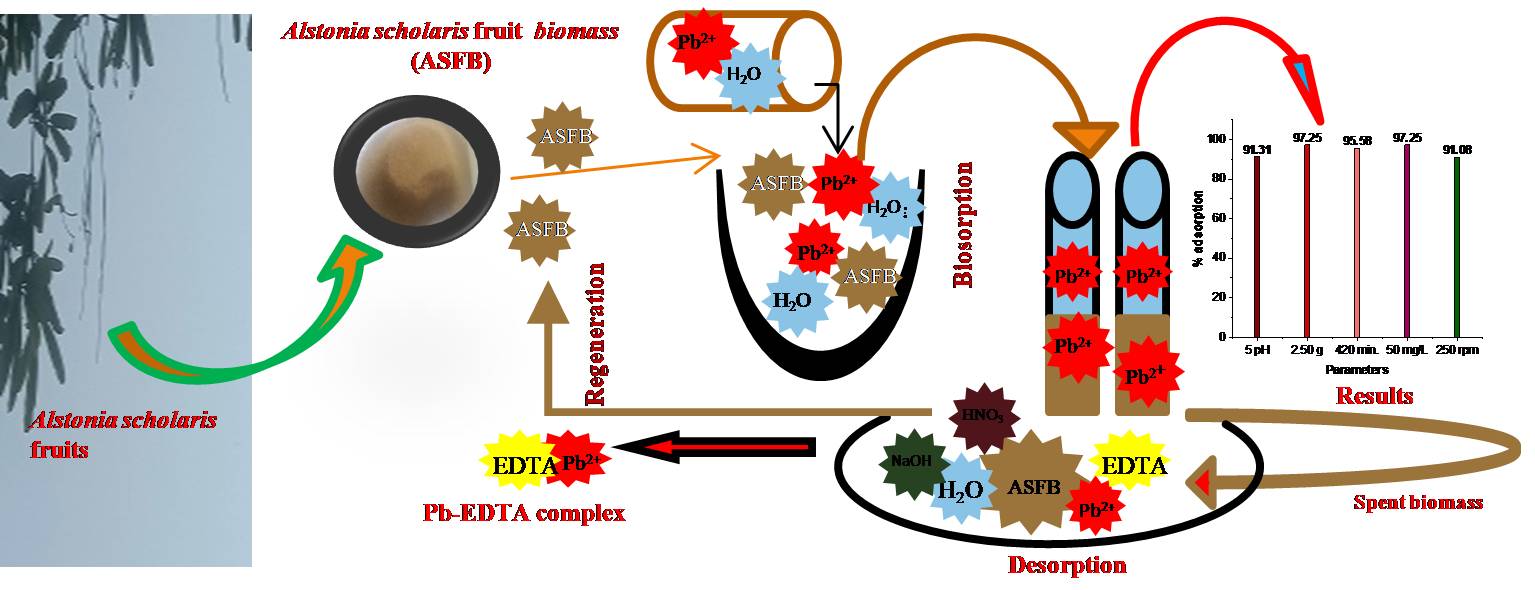
The biosorption of lead (Pb) ions on Alstonia scholaris fruit biomass was investigated in batch and column experiments in an aqueous solution, emphasising the possible binding mechanism of Pb ions on the fruit biomass. The adsorption process was found to be dependent on parameters like adsorbent dose, initial Pb ion concentration, contact time, and pH. No impact was found on Pb adsorption during competitive adsorption. The results suggested that the fruit biomass could remove more than 97% of Pb ions from the aqueous solution. The Freundlich isotherm model best explained the obtained results. The monolayer adsorption capacity of the A. scholaris fruit biomass was 50 mg g-1. The reaction rate was fast initially with most Pb adsorption taking place during the first 80 minutes, while the equilibrium was attained at 420 minutes. Scanning electron microscopy revealed changes in the surface texture of A. scholaris fruit biomass after adsorption of Pb. The biomass’s surface area was determined using Brunauer-Emmett-Teller technique. Desorption, Fourier transform infrared spectroscopy, and functional group modification revealed that the bonding of Pb ions on Alstonia scholaris fruit biomass involved complex mechanisms.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Applied Environmental Research effective when the article is accepted for publication thus granting Applied Environmental Research all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.