Minimization The Chemical Oxygen Demand (COD) Content in Tannery Wastewater Using Activated Carbon from Spent Tea Leaves
Main Article Content
Abstract
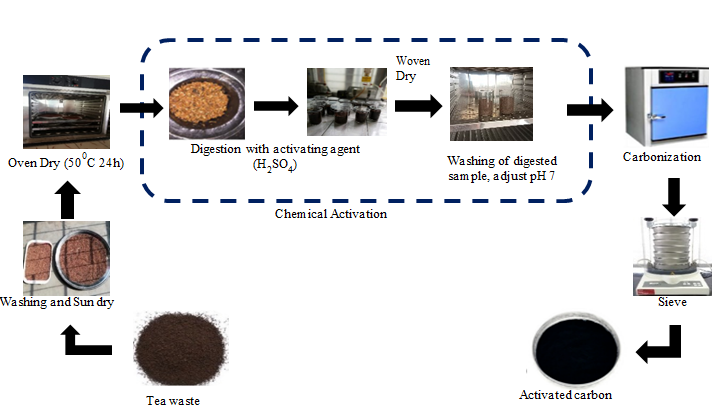
Water pollution from tannery wastewater is a serious environmental issue. This study aimed to investigate the potential of spent tea leaves as activated carbon (AC) to minimize chemical oxygen demand (COD) of tannery effluent. To determine the best conditions for minimizing COD, a batch adsorption process was conducted, involving various factors such as different adsorbent dosages (ranging from 1 to 11 gm L-1), contact time (30 to150 min), and pH values (ranging from 3 to 11). The characterization was done by Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM). The outcomes of this study demonstrated that using 1.5 M H2SO4 as activating agents resulted in iodine values of 750.52 mg g-1. Employing a higher concentration of H2SO4 had several positive effects, such as minimizing total dissolved solids (TDS) and turbidity, increasing dissolved oxygen (DO), and lowering COD in tannery wastewater. The most significant COD reduction, reaching 96.16%, was achieved with a dosage of 5 gm L-1. Furthermore, the data collected from these experiments were analyzed using the Langmuir isotherm model, which provided an excellent fit (R2 = 0.9837 and qmax = 5.186 mg g-1). Based on these result, it can be concluded that 1.5 M H2SO4 is the optimal choice for developing activated carbon from spent tea leaves. Additionally, the resulting activated carbon proves to be an effective adsorbent for minimizing COD from tannery wastewater.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Applied Environmental Research effective when the article is accepted for publication thus granting Applied Environmental Research all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Duru C. E., Enedoh M. C., Duru J. A. Surface Modification of Powdered Maize Husk, with Sodium Hydroxide for Enhanced Adsorption of Pb(II) Ions from Aqueous Solution. Journal of Environmental Treatment Techniques. 2021; 9 (1): 95-104.
Chen S., Yue Q., Gao B., Li Q., Xu X. Removal of Cr (VI) from aqueous solution using modified corn stalks: Characteristic, equilibrium, kinetic and thermodynamic study. Chemical Engineering Journal.2011 (168):909-917.
Saha B, Azam FAB. Probable Ways of Tannery’s Solid and Liquid Waste Management in Bangladesh - An Overview. Textile & Leather Review. 2021; 4(2):76-95.
Kanagaraj J, Senthilvelan T, Panda R.C., Kavitha S. Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: A comprehensive review. Journal of Cleaner Production. 2015; 89:1–17.
Nigam M., Rajoriya S., Singh S. R., Kumar P. Adsorption of Cr (VI) ion from tannery wastewater on tea waste: Kinetics, equilibrium and thermodynamic studies. Journal of Environmental Chemical Engineering.2019(7): 103188.
R. Ricky R, Shanthakumar S, Ganapathy G.P, Chiampo F. Zero Liquid Discharge System for the Tannery Industry—An Overview of Sustainable Approaches. Recycling. 2022; 7(3): 31.
Kong J, Yue Q, Huang L, Gao Y, Sun Y, Gao B, Li Q, Wang Y. Preparation, characterization and evaluation of adsorptive properties of leather waste based activated carbon via physical and chemical activation. Chemical Engineering Journal. 2013; 221(62–71).
Patel H. Charcoal as an adsorbent for textile wastewater treatment. Seperation Science and Technology. 2018; 53(17):2797–2812.
Parthasarathy P, Narayanan S.K. Effect of Hydrothermal Carbonization Reaction Parameters on the properties of hydrochar and pellets. Environmental Progress and Sustainable Energy. 2014; 33(3):676–680.
Babu B.V., Gupta S. Adsorption of Cr (VI) using activated neem leaves: kinetic studies. Adsorption.2008; 14: 85-92.
Production/Yield Quantities of Tea Leaves in world+ (Total) 1994-2021[Online]. Available: https://www.fao.org/faostat/en/#data/QCL/visualize
Statistical Bulletin of Bangladesh Tea Board for the Month of December , 2021. 2021 [Online]. Available:http://teaboard.portal.gov.bd/sites/default/files/files/teaboard.portal.gov.bd/monthly_report/3b9cb6a4_f4c5_44a0_9434_7326dc494560/2022-01-25-06-52-e62cc9dbc5e89d4c84fb045f78b983eb.pdf
Debnath B., Haldar D., Purkait M. K. Potential and sustainable utilization of tea waste: A review on present status and future trends. Journal of Environmental Chemical Engineering.2021;9(5):106179.
Islam MR, Rahman M, Haque MN. The amount of waste generated from road side shop with it consequences: case study on kuet pocket gate Fulbarigatekhulna, Bangladesh. The 4th International Conference on Civil Engineering for Sustainable Development (ICCESD 2018).9-11 February 2018;(ISBN-978-984-34-3502-6), ICCESD-2018-4377-1.
Gao P. and Ogata Y. CHAMU: An effective approach for improving the recycling of tea waste. IOP Conference Series: Material Science and Enginnering. 2020; 711(1)(012024).
Elbasiouny, H., Darwesh, M., Elbeltagy, H. et al. Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: a review. Environmental Monitoring and Assessment. 2021; 193(449).
Zhou J, Luo A, Zhao Y. Preparation and characterisation of activated carbon from waste tea by physical activation using steam. Journal of the Air & Waste Management Association. 2018; 68( 12):1269–1277.
Gurten II, Ozmak M, Yagmur E, Aktas Z. Preparation and characterisation of activated carbon from waste tea using K2CO3. Biomass and Bioenergy, 2012; 37:73–81.
Fadhil AB, Dheyab MM, Abdul-Qader AQY. Purification of biodiesel using activated carbons produced from spent tea waste. Journal of the Association of Arab Universities for Basic and Applied Sciences. 2012; 11(1):45–49, 2012.
Auta M, Hameed BH. Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chemical Engineering Journal. 2011; 171(2):502-509.
Malhotra M, Suresh S, Garg A. Tea waste derived activated carbon for the adsorption of sodium diclofenac from wastewater: adsorbent characteristics, adsorption isotherms, kinetics, and thermodynamics. Environmental Science and Pollution Research. 2018; 25(32):32210–32220.
KIRBAŞLAR Şİ, KIRBAŞLAR FG, MAHRAMANLIOĞLU M, SEVGİLİ ML, DRAMUR U. Utilization of Hazelnut Husks, Tea and Tobacco Wastes, As Raw Materials. Journal of Engineering Sciences. 2001; 7(1): 139-143
Sahu JN, Acharya J, Meikap BC. Response surface modeling and optimization of chromium(VI) removal from aqueous solution using Tamarind wood activated carbon in batch process. Journal of Hazardous Materials. 2009;172(2-3):818-25.
Mopoung S, Moonsri P, Palas W, Khumpai S. Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(III) Adsorption from Aqueous Solution. Scientific World Journal. 2015;2015:415961.
Yadavalli, R., Heggers, G.R.V.N. Two stage treatment of dairy effluent using immobilized Chlorella pyrenoidosa . Journal of Environmental Health Science & Engineering. 2013; 11(36).
Khan T, Isa MH, Ul Mustafa MR, Yeek-Chia H, Baloo L, Binti Abd Manan TS, et al. Cr(vi) adsorption from aqueous solution by an agricultural waste based carbon. RSC Advances. 2016;6(61):56365–74.
Gorzin F, Bahri Rasht Abadi M. Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorption Science & Technology. 2018;36(1-2):149-169.
Ali A, Saeed K, Mabood F. Removal of chromium (VI) from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent. Alexandria Engineering Journal. 2016 Sep;55(3):2933–42.
Ghasemi M., Ghoreyshi A.A., Younesi H., Khoshhal, S. Synthesis of a high characteristics activated carbon from walnut shell for the removal of Cr (VI) and Fe (II) from aqueous solution: single and binary solutes adsorption. Iranian Journal of Chemical Engineering. 2015; 12(4): 28-51.
Babu B.V., Gupta S. Adsorption of Cr(VI) using activated neem leaves: kinetic studies. Journal of the International Adsorption Society. 2008; 14:85–92.
Wen Y, Tang Z, Chen Y, Gu Y. Adsorption of Cr(VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent. Chemical Engineering Journal. 2011 Nov; 175:110–6.