The Effects of the Preparation Factors on the Properties of CO2 Capture Sorbent Derived from Local Waste Eggshell
Main Article Content
Abstract
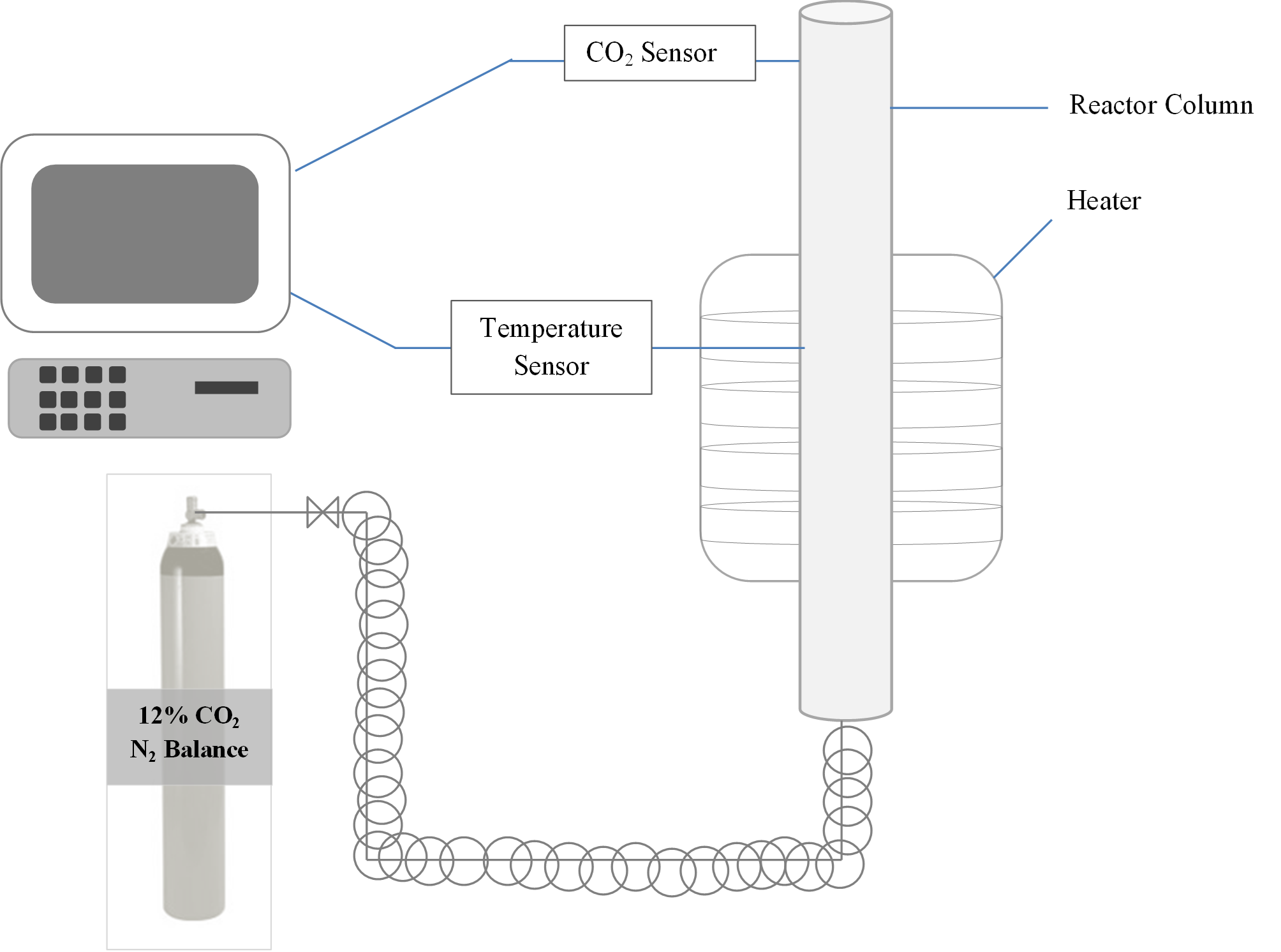
In this study, the CaO adsorbent was prepared by using local waste eggshell as a raw material. The influence of preparation parameters including calcination temperature, calcination time and particle size of eggshell powder on the adsorption surface area were investigated. By means of statistically experiment design, the interesting parameters were investigated by a full 23 factorial design. The prepared sorbent material was characterized by X–ray diffraction (XRD), Scanning electron microscopy (SEM) and the Brunauer–Emmet–Teller (BET). The results showed the particle size of eggshell powder greatly affected the BET surface area. By applying the prepared condition of 149 μm in particle size, 6 h of calcination time and 1,000oC calcination temperature, the obtained CaO adsorbent yielded the highest in BET surface area of 5.023 m2 g-1. The CO2 performance test in the fluidized bed reactor with 12% V/V CO2 in N2 balance also revealed that the local waste eggshell can be used as CO2 adsorbent. The CO2 carrying capacity of the highest BET surface area sorbent was 0.011 g CO2 g-1 sorbent at 700°C carbonation temperature.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Applied Environmental Research effective when the article is accepted for publication thus granting Applied Environmental Research all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Khajuria, A., Atienza, V.A., Chavanich, S., Henning, W., Islam, I., Kral, U., Liu, M., Liu, X., Murthy, I.K., Oyedotun, T.D.T., Verma, P., Xu, G., Zeng, X. and Li, J. Accelerating circular economy solutions to achieve the 2030 agenda for sustainable development goals. Circular Economy, 2022, 1, 100001.
Usmani, Z., Sharma, M., Awasthi, K.A., Sharma, G.D., Cysneiros, D., Nayak, S.C., Thakur, V.K., Naidu, R., Pandey, A., and Gupta, V.K. Minimizing hazardous impact of food waste in a circular economy –Advances in resource recovery through green strategies. Journal of Hazardous Materials, 2021, 416, 126154
Plaza, M.G., González, A.S., Pevida, C., Pis, J.J., Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Applied energy, 2012, 99, 272–279.
Yongping, Y., Rongrong, Z., Liqiang, D., Kavosh, M., Patchigoll, K., Oakey, J. Integration and evaluation of a power plant with a CaO - based CO2 capture system. International Journal of Greenhouse Gas Control, 2010, 4, 603–612.
Abanades, J.C., Alonso, M. and Rodriguez, N. Experimental validation of in situ CO2 capture with CaO during the low temperature combustion of biomass in a fluidized bed reactor. International Journal of Greenhouse Gas Control, 2011, 5, 512 – 520.
Hsieh, S.L., Li, F.Y., Lin, P.Y., Beck, D.E., Kirankumar, R., Wang, G.J. and Hsieh, S. CaO recovered from eggshell waste as a potential adsorbent for greenhouse gas CO2. Journal of Environmental Management, 2021, 297, 113430.
Castilho, S., Kiennemann, A., Pereira, M.F.C., Dias, A.P.S. Sorbents for CO2 capture from biogenesis calcium wastes. Chemical Engineering Journal, 2013, 226, 146–153.
Witoon, T. Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceramics International, 2011, 37, 3291–3298.
Pellerano, A., Pré, P., Kacem, M., Delebarre, A. CO2 capture by adsorption on activated carbon using pressure modulation. Energy Procedia, 2009, 1, 647–653.
Levenspiel, O. Fluid–Particle reactions: Kinetics. In: Chemical reaction engineering. New York: John Wiley & Sons, 1999. 566-588.
Hassibi, M. An overview of lime slaking and factors that affect the process. 3rd International Sorbalit symposium, New Orleans, USA, 3- 5 November 1999.
Rashidi, N.A., Mohamed, M., Yusup, S. The kinetic model of calcination and carbonation of Anadara Granosa, International Journal of Renewable energy research, 2012, 2, 497–503.
Montgomery, D. C. Experiments with a single factor: The analysis of variance. In: Design and analysis of experiments. New York: John Wiley & Sons, 2020, 55-114.