Application of Fe3O4/thiamine Magnetic Particles in the Removal of Methylene Blue
Main Article Content
Abstract
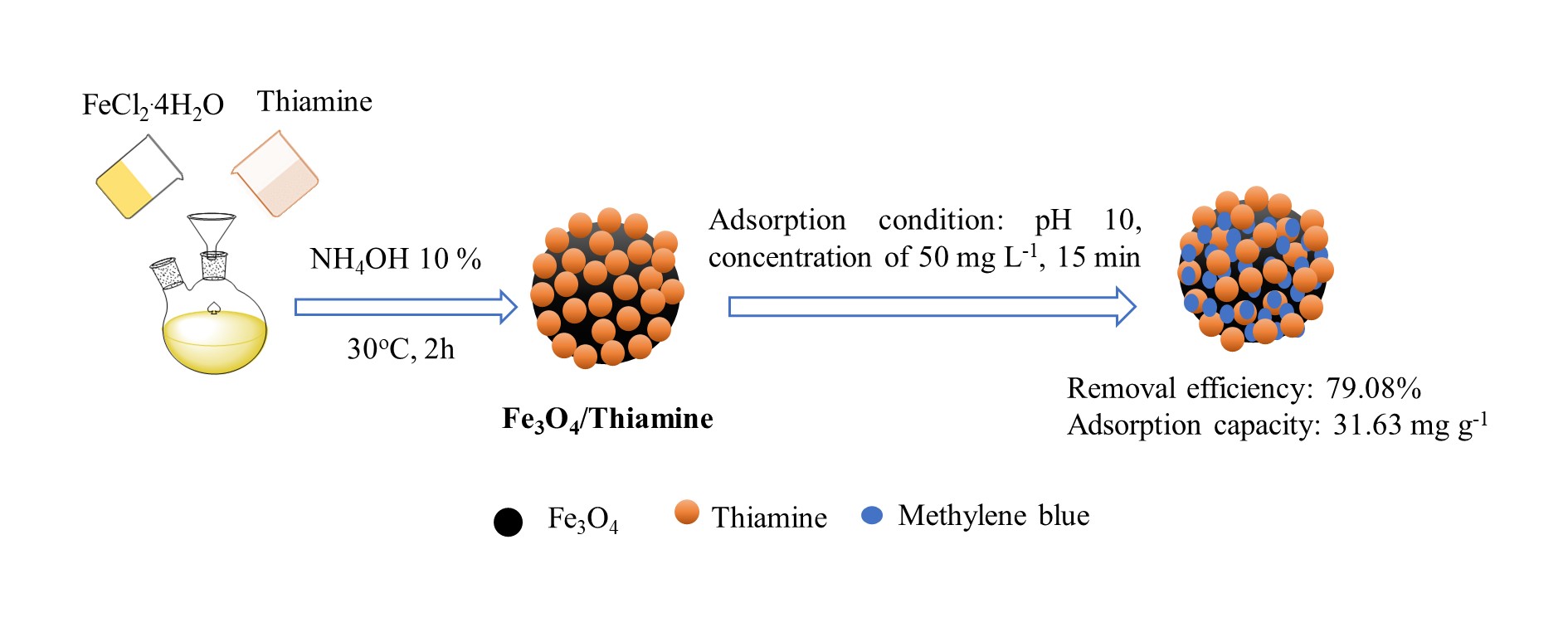
Fe3O4/thiamine particles were prepared in this work via precipitation method. The synthesis method is based on the principle of precipitation of Fe3O4 particles in the presence of thiamine coating agent. Also, the potential application of Fe3O4/ thiamine in the removal of methylene blue (MB) was investigated. Several factors that affect the synthesis of Fe3O4/thiamine such as base concentration, mass ratio of FeCl2 to thiamine, reaction temperature, and reaction time were determined. Optimal conditions for preparing Fe3O4/thiamine are NH4OH concentration = 10%, mass ratio of FeCl2:thiamine = 5:1 (g g-1), reaction temperature = 30 °C, reaction time = 120 min. The average particle size of Fe3O4/thiamine is 293.7 nm while the specific surface area, pore diameter, and magnetization of the obtained Fe3O4/ thiamine particles are 57 m2 g-1, 192.67 Å, and 2.4 emu g-1, respectively. The interesting point of this work is to obtain the Fe3O4/thiamine at low temperature with less amount of NH4OH used. Furthermore, 79.08% of MB could be removed using Fe3O4/thiamine as an adsorbent, with a maximum adsorption capacity of 31.63 mg g-1 at pH of 10, a MB concentration of 50 mg L-1, and an adsorption time of 15 min. Adsorption kinetics studies showed that the pseudo-second-order model fitted the experimental data better than the pseudo-first-order and the adsorption process is physical adsorption following the Freundlich adsorption isotherm model. An adsorption mechanism of MB onto Fe3O4/thiamine was also suggested. The synthesized Fe3O4/ thiamine particles could be a potential material for treating wastewater.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Applied Environmental Research effective when the article is accepted for publication thus granting Applied Environmental Research all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Zhang, X., Zhang, P., Wu, Z., Zhang, L., Zeng, G., Zhou, C. Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloids Surfaces A: Physicochemical Engineering Aspects, 2013, 435, 85-90.
Pirbazari, A.E., Saberikhah, E., Kozani, S.H. Fe3O4–wheat straw: preparation, characterization and its application for methylene blue adsorption. Water Resources Industry, 2014, 7, 23-37.
Yari, M., Rajabi, M., Moradi, O., Yari, A., Asif, M., Agarwal, S., Gupta, V.K. Kinetics of the adsorption of Pb (II) ions from aqueous solutions by graphene oxide and thiol functionalized graphene oxide. Journal of Molecular Liquids, 2015, 209, 50-57.
Chae, H.S., Kim, S.D., Piao, S.H., Choi, H.J. Core-shell structured Fe3O4@SiO2 nanoparticles fabricated by sol–gel method and their magnetorheology. Colloid Polymer Science, 2016, 294(4), 647-655.
Vuong, T.K.O., Le, T.L., Pham, D.V., Pham, H.N., Le Ngo, T.H., Do, H.M., Nguyen, X.P. Synthesis of high-magnetization and monodisperse Fe3O4 nanoparticles via thermal decomposition. Materials Chemistry Physics, 2015, 163, 537-544.
Liu, Y., Liu, P., Su, Z., Li, F., Wen, F. Attapulgite–Fe3O4 magnetic nanoparticles via co-precipitation technique. Applied Surface Science, 2008, 255(5), 2020-2025.
Lu, T., Wang, J., Yin, J., Wang, A., Wang, X., Zhang, T. Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surfaces A: Physicochemical Engineering Aspects, 2013, 436, 675-683.
Nabiyouni, G., Julaee, M., Ghanbari, D., Aliabadi, P.C., Safaie, N. Room temperature synthesis and magnetic property studies of Fe3O4 nanoparticles prepared by a simple precipitation method. Journal of Industrial Engineering Chemistry, 2015, 21, 599-603.
Zhang, F., Zhao, Z., Tan, R., Guo, Y., Cao, L., Chen, L., Li, J., Xu, W., Yang, Y., Song, W. Selective and effective adsorption of methyl blue by barium phosphate nano-flake. Journal of colloid interface science, 2012, 386(1), 277-284.
Yu, S., Wang, J., Cui, J. Preparation of a novel chitosan-based magnetic adsorbent CTS@SnO2@Fe3O4 for effective treatment of dye wastewater. International Journal of Biological Macromolecules, 2020, 156, 1474-1482.
Aslam, S., Zeng, J., Subhan, F., Li, M., Lyu, F., Li, Y., Yan, Z. In situ one-step synthesis of Fe3O4@MIL-100 (Fe) core-shells for adsorption of methylene blue from water. Journal of Colloid Interface science, 2017, 505, 186-195.
Mallakpour, S., Nouruzi, N. Application of vitamin B1-coated carbon nanotubes for the production of starch nanocomposites with enhanced structural, optical, thermal and Cd (II) adsorption properties. Journal of Polymers, 2018, 26(7), 2954-2963.
Liang, W., Hu, H.Y, Song, Y.D., Wang, H., Guo, Y.F. Effects of thiamine on treatment performance of textile wastewater. Desalination, 2009, 242(1-3), 110-114.
Angkawijaya, A.E., Tran-Chuong, Y.N., Ha, Q.N, Tran-Nguyen, P.L., Santoso, S.P., Bundjaja, V., Go, A.W., Hsu, H.Y., Ju, Y.H. Studies on the performance of functionalized Fe3O4 as phosphate adsorbent and assessment to its environmental compatibility. Journal of the Taiwan Institute of Chemical Engineers, 2022, 131, 104162.
Tran-Nguyen, P.L., Angkawijaya, A.E., Ha, Q.N., Tran-Chuong, Y.N., Go, A.W., Bundjaja, V., Gunarto, C., Santoso, S.P., Ju, Y.H. Facile synthesis of superparamagnetic thiamine/Fe3O4 with enhanced adsorptivity toward divalent copper ions. Chemosphere, 2022, 291, 132759.
Shaterian, H.R., Molaei, P. Fe3O4@vitamin B1 as a sustainable superparamagnetic heterogeneous nanocatalyst promoting green synthesis of trisubstituted 1, 3‐thiazole derivatives. Applied Organometallic Chemistry, 2019, 33(7), e4964.
Mittal, H., Ballav, N., Mishra, S.B. Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of methylene blue from aqueous solution. Journal of Industrial Engineering Chemistry, 2014, 20(4), 2184-2192.
Yuanbi, Z., Zumin, Q., Huang, J. Preparation and analysis of Fe3O4 magnetic nanoparticles used as targeted-drug carriers. Chinese Journal of Chemical Engineering, 2008, 16(3), 451-455.
Jia, B., Gao, L. Fabrication of Fe3O4 core-shell polyhedron based on a mechanism analogue to Ostwald ripening process. Journal of Crystal Growth, 2007, 303(2), 616-621.
Zaitsev, V.S., Filimonov, D.S., Presnyakov, I.A., Gambino, R.J., Chu, B. Physical and chemical properties of magnetite and magnetite-polymer nanoparticles and their colloidal dispersions. Journal of Colloid Interface Science, 1999, 212(1), 49-57.
Yan, H., Zhang, J., You, C., Song, Z., Yu, B., Shen, Y. Influences of different synthesis conditions on properties of Fe3O4 nanoparticles. Materials Chemistry Physics, 2009, 113(1), 46-52.
Zhang, H., He, X., Zhao, W., Peng, Y., Sun, D., Li, H., Wang, X. Preparation of Fe3O4/TiO2 magnetic mesoporous composites for photocatalytic degradation of organic pollutants. Water Science Technology, 2017, 75(7), 1523-1528.
Rafiee, F., Mehdizadeh, N. Palladium n-heterocyclic carbene complex of vitamin B1 supported on silica-coated Fe3O4 nanoparticles: A green and efficient catalyst for c–c coupling. Catalysis Letters, 2018, 148(5), 1345-1354.
Li, W., Zhang, B., Li, X., Zhang, H., Zhang, Q. Preparation and characterization of novel immobilized Fe3O4@SiO2@mSiO2–Pd (0) catalyst with large pore-size mesoporous for Suzuki coupling reaction. Applied Catalysis A: General, 2013, 459, 65-72.
Bao, X., Qiang, Z., Chang, J.H., Ben, W., Qu, J. Synthesis of carbon-coated magnetic nanocomposite (Fe3O4@C) and its application for sulfonamide antibiotics removal from water. Journal of Environmental Sciences, 2014, 26(5), 962-969.
Zazouli, M.A., Ghanbari, F., Yousefi, M., Madihi-Bidgoli, S. Photocatalytic degradation of food dye by Fe3O4–TiO2 nanoparticles in presence of peroxymonosulfate: The effect of UV sources. Journal of Environmental Chemical Engineering, 2017, 5(3), 2459-2468.
Xuan, S., Wang, F., Lai, J.M., Sham, K.W., Wang, Y.X.J., Lee, S.F., Yu, J.C., Cheng, C.H., Leung, K.C.F. Synthesis of biocompatible, mesoporous Fe3O4 nano/microspheres with large surface area for magnetic resonance imaging and therapeutic applications. ACS applied materials interfaces, 2011, 3(2), 237-244.
Yimin, D., Jiaqi, Z., Danyang, L., Lanli, N., Liling, Z., Yi, Z., Xiaohong, Z. Preparation of Congo red functionalized Fe3O4@SiO2 nanoparticle and its application for the removal of methylene blue. Colloids Surfaces A: Physicochemical Engineering Aspects, 2018, 550, 90-98.
Zhang, Z., Zhao, X., Jv, X., Lu, H., Zhu, L. A simplified method for synthesis of l-tyrosine modified magnetite nanoparticles and its application for the removal of organic dyes. Journal of Chemical Engineering Data, 2017, 62(12), 4279-4287.
Ghaedi, M., Hassanzadeh, A., Kokhdan, S.N. Multiwalled carbon nanotubes as adsorbents for the kinetic and equilibrium study of the removal of alizarin red S and morin. Journal of Chemical Engineering Data, 2011, 56(5), 2511-2520.
Song, N., Wu, X.L., Zhong, S., Lin, H., Chen, J.R. Biocompatible G-Fe3O4/CA nanocomposites for the removal of Methylene Blue. Journal of Molecular Liquids, 2015, 212, 63-69.
Saini, J., Garg, V., Gupta, R. Removal of methylene blue from aqueous solution by Fe3O4@Ag/SiO2 nanospheres: synthesis, characterization and adsorption performance. Journal of Molecular Liquids, 2018, 250, 413-422.
Ma, J., Yu, F., Zhou, L., Jin, L., Yang, M., Luan, J., Tang, Y., Fan, H., Yuan, Z., Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Applied Materials Interfaces, 2012, 4(11), 5749-5760.
Xie, Y., Qian, D., Wu, D., Ma, X. Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chemical Engineering Journal, 2011, 168(2), 959-963.
Chang, J., Ma, J., Ma, Q., Zhang, D., Qiao, N., Hu, M., Ma, H., Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Applied Clay Science 2016, 119, 132-140.