Reduction of Interference Ions in Spent Plating Solution for the Chromium Electroplating Process
Abstract
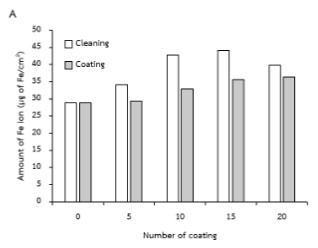
Hard chrome electroplating processes are usually cause by iron(III) ions and chromium(III) ion contaminants problems. The plating solution is required to contain no more than 15 g/L of interfering ions. The similar conditions for electroplating process of the industrial establishments were studied in this research, in the topic of iron ion leaching rate and sludge occurring in solution. Anode was an alloy of Sn and Pb and cathode was a 1 inch2 mild steel specimen which applied current at 3.0 A at 50 °C for thickness of 10±3 µm. Monitoring after electroplating process 1-20 times, it was found that the amount of iron ions contents and sludge increasing correlated with number of electroplating times. Primary troubleshooting was the separation of plating pond for the surface cleaning process from the electroplating process. In addition, the reduction of chromium(III) ion contamination was the oxidation of chromium(III) ion to dichromate ion by adding the excess of persulphate ion with heating. Chromium (III) ions converted to dichromate were obviously noticeable with green to be orange color. The remaining of persulphate after the oxidation was eliminated to hydrogen sulphate, that’s not interfere the system because all were containing in spent solution.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2022 The Journal of Applied Science

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

