Adsorption Efficiency, Adsorption Isotherms and Kinetic Study for Methylene Blue Removal in Aqueous Solution of Thiol-Functionalized Mesoporous Silica Nanospheres
Main Article Content
Abstract
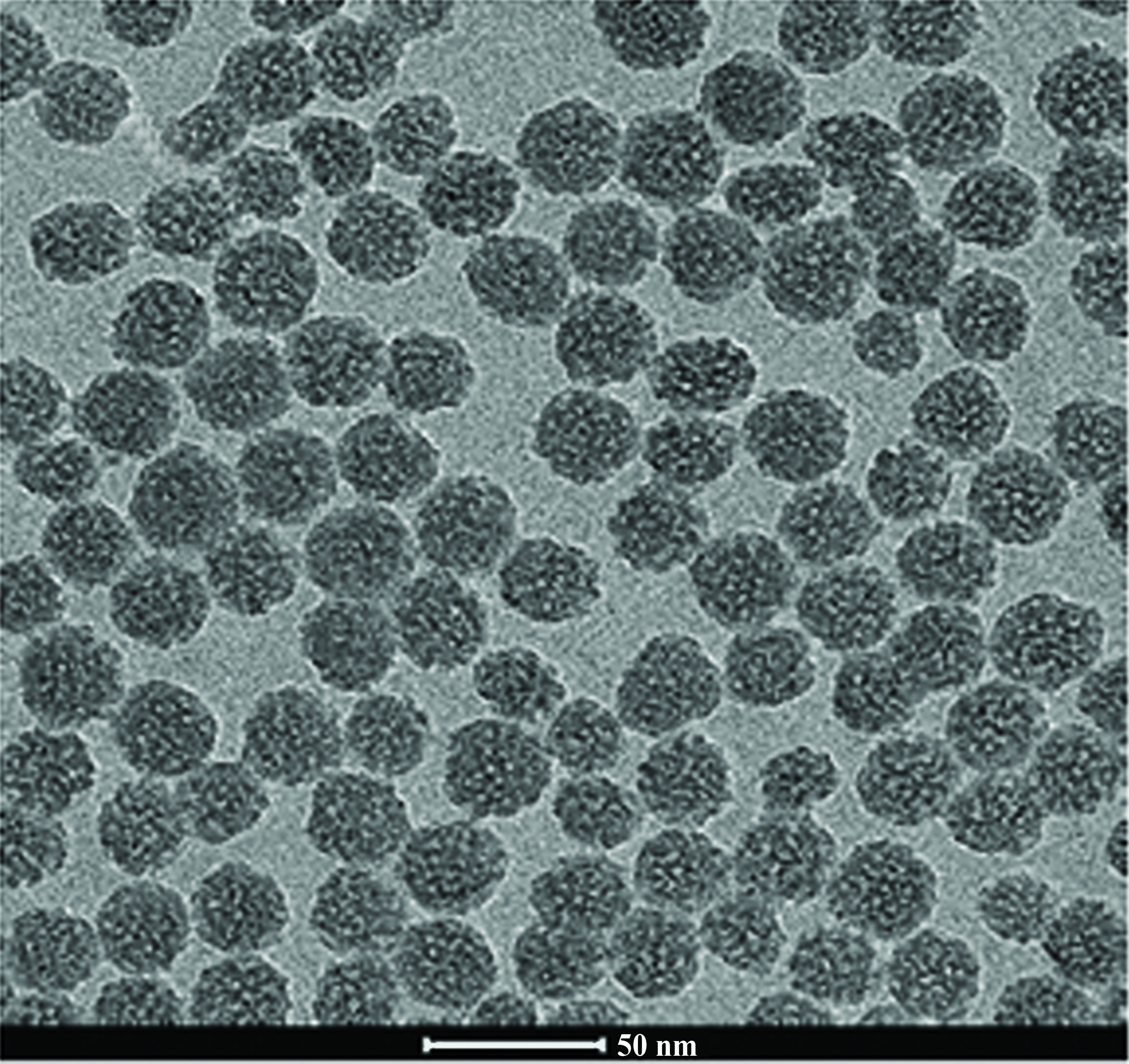
Thiol-functionalized mesoporous silica nanospheres material was synthesized by co-condensation method under biphasic system. Structural characterization comfirmed the mesoporous structure and the content of adsorbents by transmission electron microscopy, N2 adsorption-desorption and Fourier transform inferred spectroscopy. The adsorbent were nanosphere with particle diameter lower than 50 nm. A batch adsorption study was carried out with variable pH, adsorption time, adsorbent dose, shaking rate, temperature and adsorbate concentration. Methylene blue could be removed by 0.05 g of MSN and MSN-SH at pH 10 and 30 min of adsorption time. The adsorption process followed pseudo-second-order kinetics. The experimental adsorption isotherm was found to be best fitted with the Langmuir model, which implied that the adsorption of MB as a monolayer.
Article Details
References
[2] Bhattacharyya, K. G. and Sharma, A. (2005). Kinetic and Thermodynamics of Methylene Blue Adsorption on Neem (Azadirachta indica) Leaf Powder. Dyes and Pigments. Vol. 65, Issue 1, pp. 51-59. DOI: 10.1016/j.dyepig.2004.06.016
[3] Derakhshan, Z., Baghapour, M. A., and Ranjbar, M. M. (2013). Faramarzian, Adsorption of Methylene Blue Dye from Aqueous Solutions by Modifi ed Pumice Stone: Kinetics and Equilibrium Studies. Health Scope. Vol. 2, Issue 3, pp. 136-144. DOI: 10.17795/jhealthscope-12492
[4] Han, X., Chu, L., Liu, S., Chen, T., Ding, C., Yan, J., Cui, L., and Quan, G. (2015). Removal of Methylene Blue from Aqueous Solution Using Porous Biochar Obtained by KOH Activation of Peanut Shell Biochar. Bio Resources. Vol. 10, No. 2, pp. 2836-2849. DOI: 10.15376/biores.10.2.2836-2849
[5] El Qada, E. N., Allen, S. J., and Walker, G. M. (2006). Adsorption of Methylene Blue Onto Activated Carbon Produced from Steam Activated Bituminous Coal: A Study of Equilibrium Adsorption Isotherm. Chemical Engineering Journal. Vol. 124, Issue 1-3, pp. 103-10. DOI: 10.1016/j.cej.2006.08.015
[6] Rostamian, R., Najafi , M., and Rafati, A. A. (2011). Synthesis and Characterization of Thiol-Functionalized Silica Nano Hollow Sphere as a Novel Adsorbent for Removal of Poisonous Heavy Metal Ions from Water Kinetics, Isotherms and Error Analysis. Chemical Engineering Journal. Vol. 171, Issue 3, pp. 1004-1011
[7] Castillo, X., Pizarro, J., Ortiz, C., Cid, H., Flores, M., Canck, E. D., and Voot, P. V. D. (2018). A Cheap Mesoporous Silica from Fly Ash as an Outstanding Adsorbent for Sulfate in Water. Microporous and Mesoporous Matetials. Vol. 272, pp. 184-192. DOI: 10.1016/j.micromeso.2018.06.014
[8] Yang, H., Xu, R., Xue, X., Li, F., and Li, G. (2008). Hybrid Surfactant-Templated Mesoporous Silica Formed in Ethanol and Its Application for Heavy Metal Removal. Journal of Hazardous Materials. Vol. 152, Issue 2, pp. 690-698. DOI: 10.1016/j.jhazmat.2007.07.060
[9] Li, G., Zhao, Z., Liu, J., and Jiang, G. (2011). Effective Heavy Metal Removal from Aqueous Systems by Thiol Functionalized Magnetic Mesoporous Silica. Journal of Hazardous Materials. Vol. 192, Issue 1, pp. 277-283. DOI: 10.1016/j.jhazmat.2011.05.015
[10] Li, G., Zhao, Z., Liu, J., and Jiang, G. (2013). Kinetic Studies on the Adsorption of Methylene Blue Onto Vegetal Fiber Activated Carbons. Applied Surface Science. Vol. 282, pp. 52-59. DOI: 10.1016/j.apsusc.2013.05.031
[11] Dong, C., Zhang, F., Pang, Z., and Yang, G. (2016). Efficient and Selective Adsorption of Multi-Metal Ions Using Sulfonated Cellulose as Adsorbent. Carbohydrate Polymers. Vol. 151, pp. 230-236. DOI: 10.1016/j.carbpol.2016.05.066
[12] Das, B., Mondal, N. K., Roy, P., and Chattaraj, S. (2013). Equilibrium, Kinetic and Thermodynamic Study on Chromium (VI) Removal from Aqueous Solution Using Pistia stratiotes Biomass. Chemical Science Transactions. Vol. 2, Issue 1, pp. 85-104. DOI:10.7598/cst2013.318