A simple preparation of graphene oxide with a modified Hummer's method
DOI:
https://doi.org/10.55674/ias.v12i3.251664Keywords:
Graphite, Graphene Oxide, Hummer’s methodAbstract
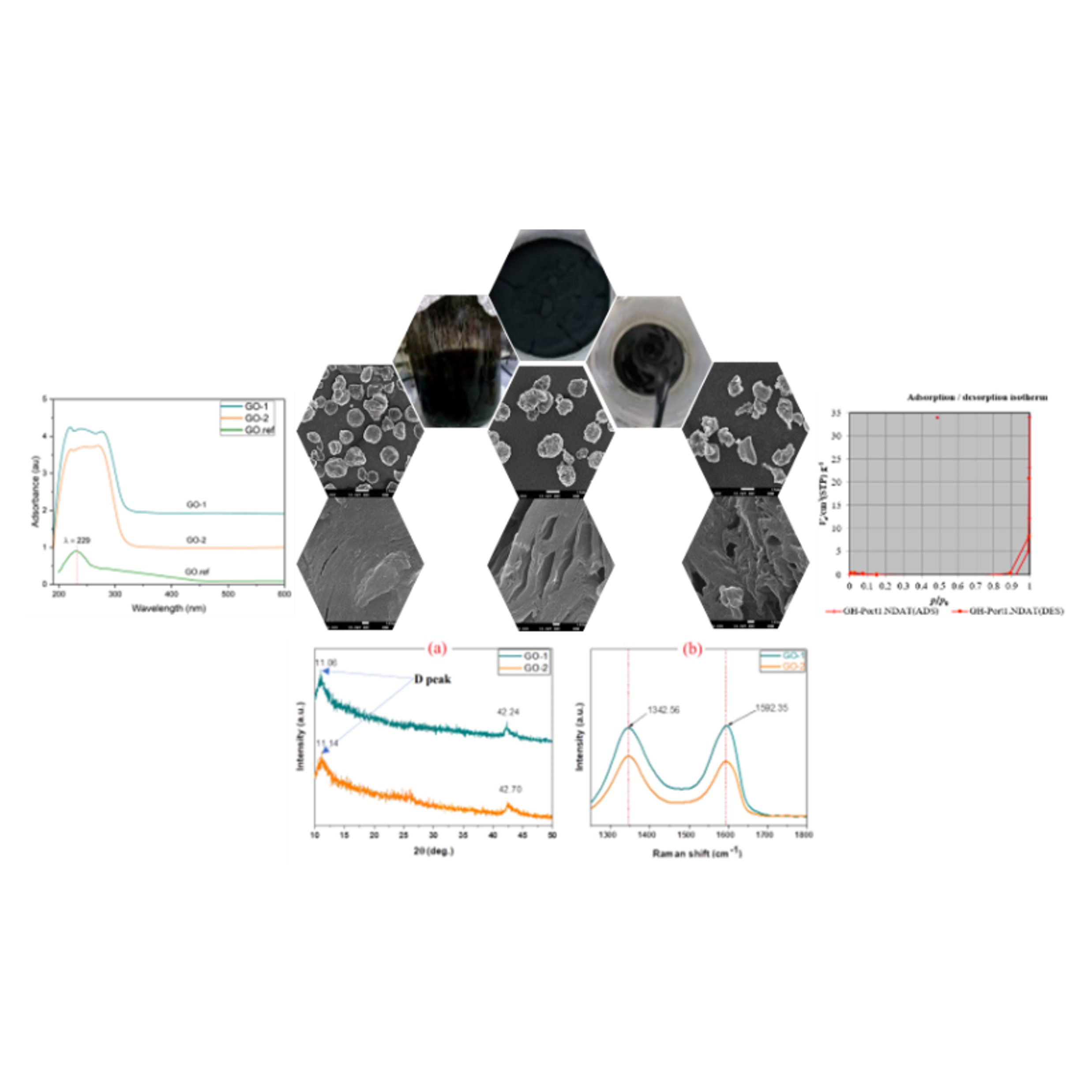
Graphene oxide (GO) has gained a lot of interest in recent years as a key precursor and a derivative of graphene. The GO was retrieved from graphite flakes using a modified Hummer's approach that differed from the normal Hummer's method. GO-method 1 using sodium nitrate and GO-method 2 not using sodium nitrate (NaNO3). Used approach is chemical exfoliation, where chemicals are used to intercalate between the graphene layers and expand the interlayer spacing. This expansion weakens the van der Waals forces, making it easier to separate the layers. GO-method 1 and GO-method 2 run on ultrasound and dry. Increasing the ultrasonic process of the procedure reduces the preparation time for graphene oxide compared to the original Hummer’s method. The X-Ray Diffractometer result of GO-method 2 shows the diffraction peaks at 2q at 11.06˚ and 42.24˚ which corresponds to graphene oxide. Raman spectroscopy and UV-visible spectrophotometry were used to investigate the molecular structure and optical properties of graphene oxide, respectively. Field Emission Scanning Electron Microscope of GO to perform elemental mapping on graphene samples and Brunauer Emmet Teller (BET) for surface area determination and pore size distribution results found that GO-method 1 and GO-method 2 corresponds to graphene oxide, which has a macroporous size, and the adsorption response is more respectively.
References
P. R. Edward, D. A .C. Brownson, C. E. Banks, A decade of graphene research: production, applications and outlook, Mater. Today. 17(9)(2014) 426 – 432.
G. Wypych, Graphene Important Results and Applications, Ontario, Canada: ChemTec Publishing. (2019).
J. I. Paredes, S. Villar-Rodil, M. J. Fernández-Merino, L. Guardia, A. Martínez-Alonso, J. M. D. Tascón, Environmentally friendly approaches toward the mass production of processable graphene from graphite oxide, J. Mater. Chem. 21 (2011) 298 – 306.
S. K. Tiwari, S. Sahoo, N. Wang, A. Huczko, Graphene research and their outputs: Status and prospect, J. Sci.: Adv. Mater. Devices. 5(1) (2020) 10 – 29.
A. A. Olorunkosebi, M. A. Eleruja, A. V. Adedeji, B. Olofinjana, O. Fasakin, E. Omotoso, K. O. Oyedotun, E. O. B. Ajayi, N. Manyala, Optimization of graphene oxide through various Hummers' methods and comparative reduction using green approach, Diam. Relat. Mater. (2021) 108456.
E. P. Randviir, D. A. C. Brownson, C. E. Banks, A decade of graphene research:production, applications and outlook, Mater. Today. 117 (2014) 426 – 432.
W. S. Hummers Jr, R. E. Offeman, Preparation of Graphitic Oxide, J. Am. Chem. Soc. 80(6) (1957), 1339 – 1339.
J. I. Paredes, S. Villar-Rodil, M. J. Fernández-Merino, L. Guardia, A. Martínez-Alonsoa, J. M. D. Tascóna, Environmentally friendly approaches toward the mass production of processable graphene from graphite oxide, J. Mater. Chem. (2011) 298 – 306.
W. Ananpreechakorn, A. Khrueakham, Preparation of activated carbon from Phu Phan Dendrocalamus asper backer, Asia Pac J Sci Technol. 26(01) (2021) APST–26.
J. Aixart, F. Díaz, J. Llorca, J. Rosell-Llompart, Increasing reaction time in Hummers’ method towards well exfoliated graphene oxide of low oxidation degree, Ceram. Int. (2021), 22130 – 22137.
N. I. Zaaba, K. L. Foo, U. Hashim, S. J. Tan, W. -W. Liu, C. H. Voon, Synthesis of Graphene Oxide using Modified Hummers Method: Solvent, Adv. Mater. Process. Technol. (2017) 469 – 477.
P. Feicht, R. Siegel, H. T. Thurn, J. W. Neubauer, M. Seuss, T. Szabó, A. V. Talyzin, C.E. Halbig, S. Eigler, D. A. Kunz, A. Fery, G. Papastavrou, J. Senker, J. Breu, Systematic evaluation of different types of graphene oxide in respect to variations in their in-plane modulus, Carbon. (2017) 700 – 705.
S. K. Tiwari, S. Sahoo, N. Wang, A. Huczko, Graphene research and their outputs: Status and prospect, Journal of Science: Advanced Materials and Devices. (2020) 10 – 29.
G. Wypych, Graphene Important Results and Applications, Ontario, Canada: ChemTec Publishing. (2019).
X. Qiao, S. Liao, C. You, R. Chen, Phosphorus and Nitrogen Dual Doped and Simultaneously Reduced Graphene Oxide with High Surface Area as Efficient Metal-Free Electrocatalyst for Oxygen Reduction, catalysts. (2015), 981 – 991.
M. S. Roslan, K. T. Chaudary, Z. Haider, A. F. M. Zin, J. Ali, Effect of magnetic field on carbon nanotubes and graphene structure synthesized at low pressure via arc discharge process, International Conference on Plasma Science and Applications (ICPSA 2016).
A. Y. Lee, K. Yang, N. D. Anh, C. Park, S. M. Lee, T. G. Lee, M. S. Jeong, Raman study of D* band in graphene oxide and its correlation with reduction, Appl. Surf. Sci. 536 (2021) 147990.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Materials Science and Applied Energy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







