Alkaline concentration on graphite hydrogen production
DOI:
https://doi.org/10.55674/ias.v12i1.247907Keywords:
Electrolyte, Hydrogen production, Voltage, Sodium hydroxideAbstract
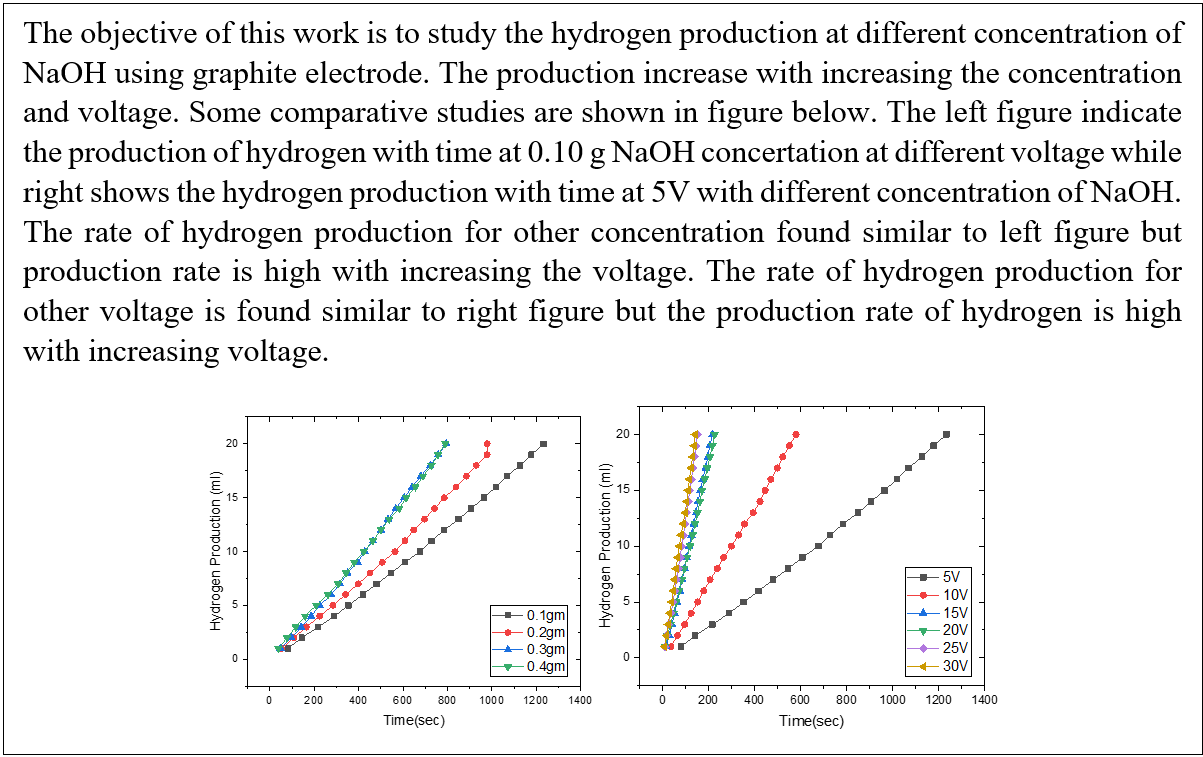
We set up the experiment and prepared four electrolytes, each containing 0.10, 0.20, 0.30, and 0.40 g of sodium hydroxide in 400 ml. The experiment was conducted at 5, 10, 15, 20, 25, and 30 V from each of the four different electrolyte concentrations. For each sample, the commutative hydrogen production is notated/measured up to 20 ml, and the time for 20 ml hydrogen production is noted. The results show that at 5 V, the time required to produce 20 ml of hydrogen is longer, whereas at 30 V, the time required is shorter, regardless of concentration. Furthermore, when considering voltage, the time required to produce 20 ml of hydrogen from 0.40 g of electrolyte takes less time than 0.10 g.
References
O.M. Fátima, M.V. Teleginski, V. Fernanda, J.V.A. Souza, Graphite Electrodes for Hydrogen Production by Acid Electrolysis, Mater. Sci. Forum. 1012 (2020) 158 − 163.
S.K. Mazloomi, N. Sulaiman, Influencing factors of water electrolysis electrical efficiency, Renew. and Sustain. Energy Rev. 16(6) (2012) 4257 − 4260.
R. Yıldız, B.D. Mert, T. Karazehir, Y. Gurdal, S.T. Doslu, Experimental and theoretical study on hydrogen production by using Ag nanoparticle-decorated graphite/Ni cathode, Inter. J. Energy Res. 45(3) (2020) 1 − 13.
H. Radinger, A. Ghamlouche, H. Ehrenberg, F. Scheiba, Origin of the catalytic activity at graphite electrodes in vanadium flow batteries, J. Mater. Chem. A. 9 (2021) 18280 − 18283.
I.T. Kato, M. Kubota, N. Kobayashi, Y. Suzuoki, Effective utilization of by-product oxygen from electrolysis hydrogen production, Energy. 30 (2005) 2580 − 2595.
M.K. Saklin, R.C. Das, Y. Akther, S. Dewanjee, S.K. Das, T.S.B. Monir, S. Mondal, Efficiency of Aluminium and Copper Coated Aluminium Electrode in Hydrogen Fuel Generation from Rain Water, Energy and Power Eng. 12 (2020) 348 − 356.
M. Zhang, J. Guan, Y. Tu, S. Wang, D. Deng, Highly efficient conversion of surplus electricity to hydrogen energy via polysulfides redox, The Inno. 2(3) (2021) 1 − 2.
S. Wang, A. Lu, C.J. Zhong, Hydrogen production from water electrolysis: role of catalysts, Nano Conver. 8(4) (2021) 1 − 3.
I.G. David, D.E. Popa, M. Buleandra, Pencil Graphite Electrodes: A Versatile Tool in Electroanalysis, J. Analyt. Methods in Chem. 1905968 (2017) 1 − 22.
A.L. Yuvaraj, D. Santhanaraj, A Systematic Study on Electrolytic Production of Hydrogen Gas by Using Graphite as Electrode, Mater. Res. 17(1) (2014) 83− 87.
C. Ho, Hydrolytic reaction of waste aluminum foils for high efficiency of hydrogen generation, Int. J. Hydro. Energy. 42(31) (2017) 19622 − 19628.
H. Zou, S. Chen, Z. Zhao, W. Lin, Hydrogen production by hydrolysis of aluminium, J. Alloys Comp. 578 (2013) 380 − 384.
K.K. Phung, S. Sethupathi, C.S. Piao, Production of H2 from aluminium/water reaction and its potential for CO2 methanation, IOP Conf. Series: Earth and Envi. Sci. 140 (012020) (2018) 3 − 4.
American Chemical Society, Pencil Electrolysis, Terrific Sci. Press, (2008) 1 − 14.
N. Alam, K.M. Pandey, Experimental Study of Hydroxy Gas (HHO) Production with Variation in Current, Voltage and Electrolyte Concentration, IOP Conf. Ser.: Mater. Sci. and Eng. 225 (012197) (2017) 1 − 10.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Journal of Materials Science and Applied Energy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







