Fabrication of activated carbon electrode synthesized from sacred lotus leaf natural materials for supercapacitors
DOI:
https://doi.org/10.55674/ias.v11i3.247369Keywords:
Carbon electrode, Supercapacitors, Sacred lotus leafAbstract
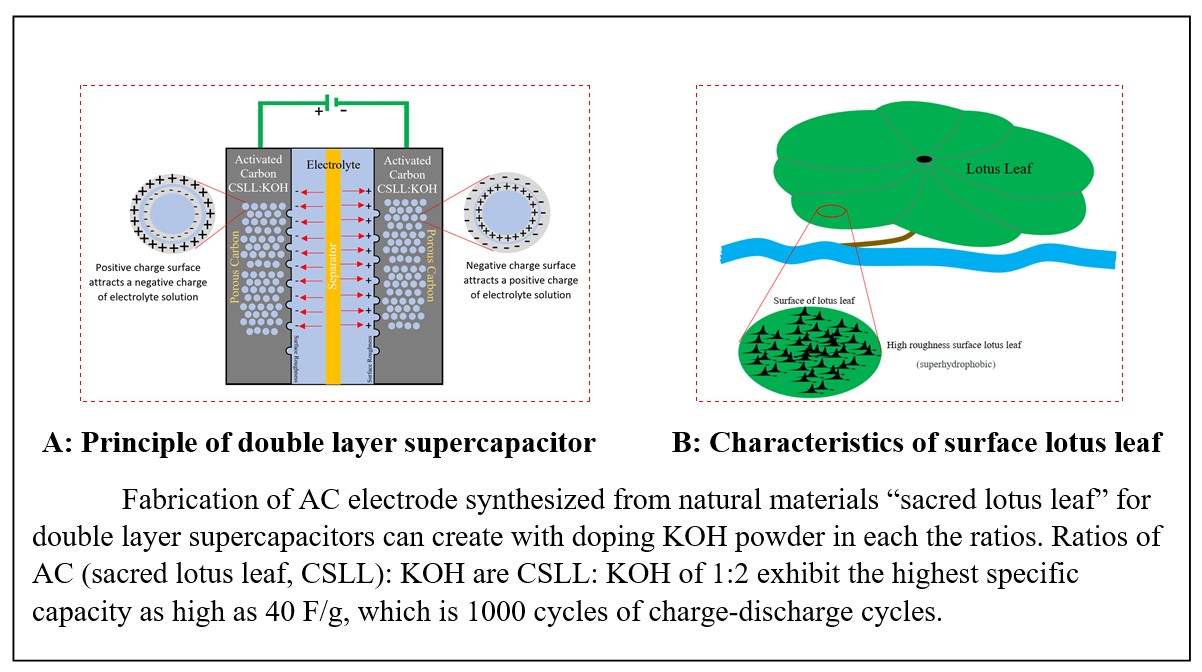
Supercapacitor has been the interesting issue in electric energy storage system. Supercapacitors carbon electrode was synthesized from a sacred lotus leaf. The none activated carbon sacred lotus leaf powder (CSLL) and the carbon sacred lotus leaf were mixed with potassium hydroxide (KOH) in the ratio of 1 : 1, 1 : 2, and 1 : 3 which were called CSLL, CSLL-1 : 1, CSLL-1 : 2 and CSLL-1 : 3, respectively. The structural, morphological properties and element component were analyzed with x-ray diffraction (XRD) technique, the field emission scanning electron microscope (FESEM) and energy dispersive x-ray spectroscopy (EDX), respectively. Electrical properties were measured by cyclic voltammetry (CV) and charge–discharge techniques. JCPDS 01-072-2091 data file confirmed the carbon-like (110) plan at 2 theta of 29.43° CSLL-1 : 1 and CSLL-1 : 2 showed high crystalline sizes. Morphology of CSLL-1 : 1 and CSLL-1 : 2 samples exhibited corrosion of surface clearly nevertheless carbon cluster adhered continuously on surface affect to higher the surface area. Carbon element of CSLL, CSLL-1 : 1 and CSLL-1 : 2 samples were obtained as high as of 74.50, 79.30 and 76 % by atomic, respectively which it was suitable characteristic of activated carbon electrode. The highest specific capacitance of CSLL-1 : 2 electrodes displayed approximately 40.85 F g-1 at the scan rate of 20 mVs-1. Moreover, the charge–discharge time of CSLL-1 : 1 and CSLL-1 : 2 electrodes showed the long discharge time more than the discharge time of CSLL-1 : 3 and CSLL electrodes. The performances of electrode demonstrated with charge-discharge of 1,500 and 1,000 cycles found that the CSLL-1 : 1 and CSLL-1 : 2 electrodes exhibited high stability. The suitable conditions ranges depicted between from the CSLL-1 : 1 to CSLL-1 : 2 ratios; furthermore, a sacred lotus leaf can fabricate the carbon electrode for supercapacitor.
References
B.K. Kim, S. Sy, A. Yu, J. Zhang, Electrochemical Supercapacitors for Energy Storage and Conversion, Handbook of Clean Energy Systems, John Wiley & Sons, Ltd. (2015) 1 – 25.
W. Raza, F. Ali, N. Raza, Y. Luo, K.H. Kim, J. Yang, S. Kumar, A. Mehmood, E.E. Kwon, Recent advancements in supercapacitor technology, Nano Energy. 52 (2018) 441 – 473.
Z.S. Iro, C. Subramani, S.S. Dash, A Brief Review on Electrode Materials for Supercapacitor, Int. J. Electrochem. Sci. 11 (2016) 10628 – 10643.
H. Ji, X. Zhao, Z. Qiao, J. Jung, Y. Zhu, Y. Lu, L.L. Zhang, H. Allan, M. Donald, S.R. Ruoff, Capacitance of carbon-based electrical double-layer capacitors, Nat. Commun. 5(1) (2014) 3317.
S. Qu, J. Wan, C. Dai, T. Jin, F. Ma, Promising as high-performance supercapacitor electrode materials porous carbon derived from biological lotus leaf, J. Alloys Comp. 751 (2018) 107 – 116.
M. Fujishige, I. Yoshida, Y. Toya, Y. Banba, Preparation of activated carbon from bamboo-cellulose fiber and its use for EDLC electrode material, J. Environ. Chem. Eng. 5 (2017) 1801 – 1808.
B. Lu, L. Hu, H. Yin, X. Mao, W. Xiao, D. Wang, Preparation and application of capacitive carbon from bamboo shells by one step molten carbonates carbonization, Int. J. Hydrog. Energy. 41 (2016) 18713 – 18720.
S. Li, K. Han, P. Si, J. Li, C. Lu, High–performance Activated Carbons Prepared by KOH Activation of Gulfweed for Supercapacitors, Int. J. Electrochem. Sci. 13 (2018) 1728 – 1743.
Q. Tian, X. Wang, X. Xu, M. Zhang, L. Wang, X. Zhao, Z. An, H. Yao, J. Gao, A novel porous carbon material made from wild rice 267 – 276.
S. Qu, J. Wan, C. Dai, T. Jin, F. Ma, Promising as high-performance supercapacitor electrode materials porous carbons derived from biological lotus leaf, J. Alloys Compd. 751 (2018) 107 – 116.
G. Zhang, Y. Chen, Y. Chen, H. Guo, Activated biomass carbon made from bamboo as electrode material for supercapacitors, Mater. Res. Bull. 102 (2018) 391 – 398.
Z. Dai, C. Peng, J.H. Chae, K.C. Ng, G.Z. Chen, Cell Voltage Versus Electrode Potential Range in Aqueous Supercapacitors, Sci. Rep. 5 (2015) 1 – 8.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Journal of Materials Science and Applied Energy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







