Chemical and Surface Properties of Activated Carbon from Banana Peel by Dry Chemical Activation
DOI:
https://doi.org/10.55674/ias.v10i3.243896Keywords:
Activated carbon, Banana peel, ZnCl2, Dry chemical activationAbstract
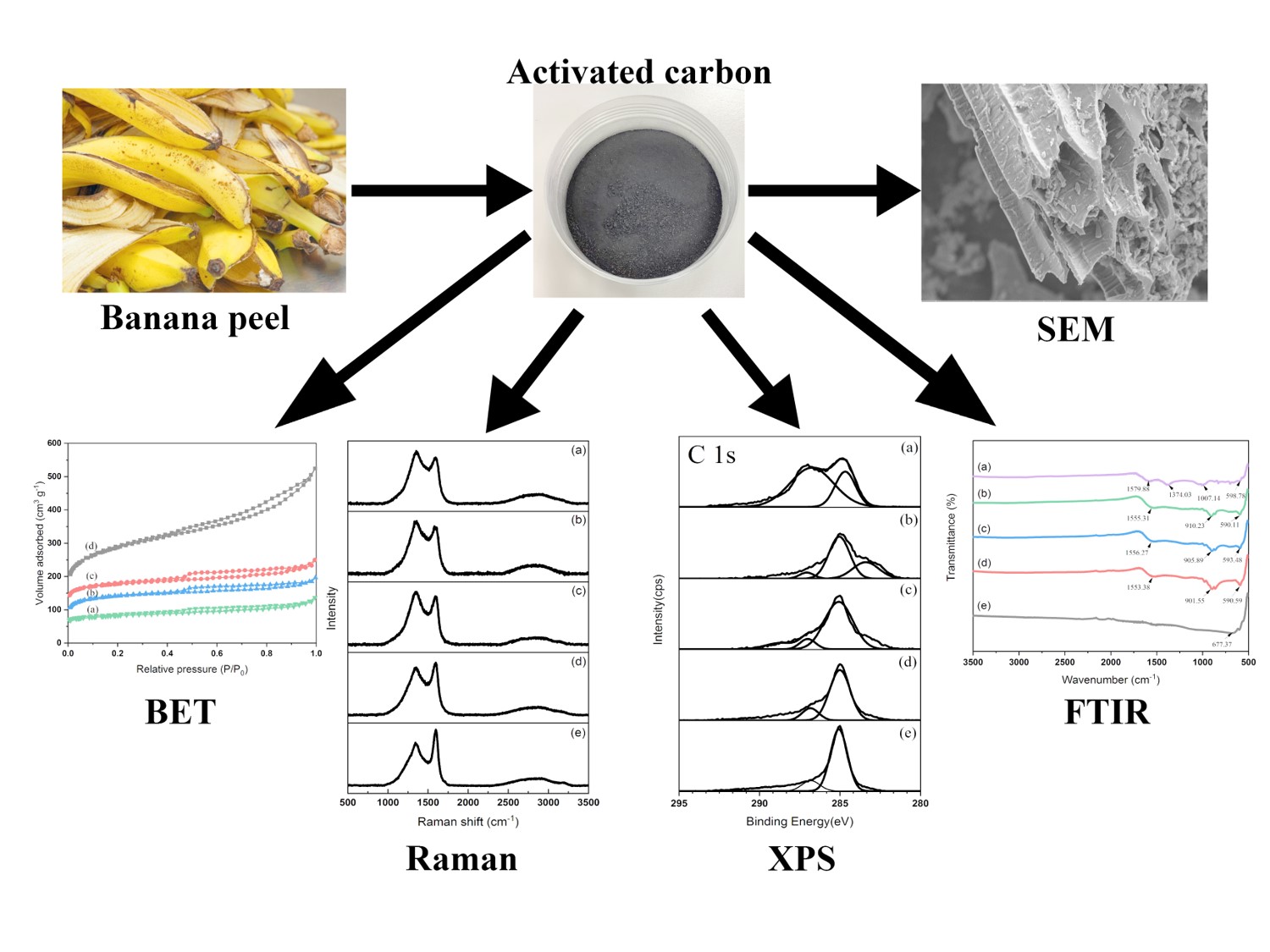
Throughout this research, chemical and surface properties of activated carbon were examined by taking into consideration dry chemical activation combined with ZnCl2 at the weight ratios between charcoal and ZnCl2 of 1:1, 1:2, 1:3 and 1:4 by activation temperature of 500 °C, 600 °C and 700 °C. The prepared activated carbon was then characterized using iodine number test method, Brunauer-Emmett-Teller (BET) surface area, Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). The results showed that the optimum ratio and temperature was found at 1:3 and 700 °C, respectively. Which indicated improved efficiency of activated carbon prepared from banana peels.