Simple Synthesis and Analysis of 3,6 and 2,7-dibromo-9-dodecylcarbazole by Direct Probe-Atmospheric Pressure Chemical Ionization Mass Spectrometry (DP-APCI-MS)
Keywords:
3,6-substituted carbazole, 2,7-substituted carbazole, DP-APCI-MSAbstract
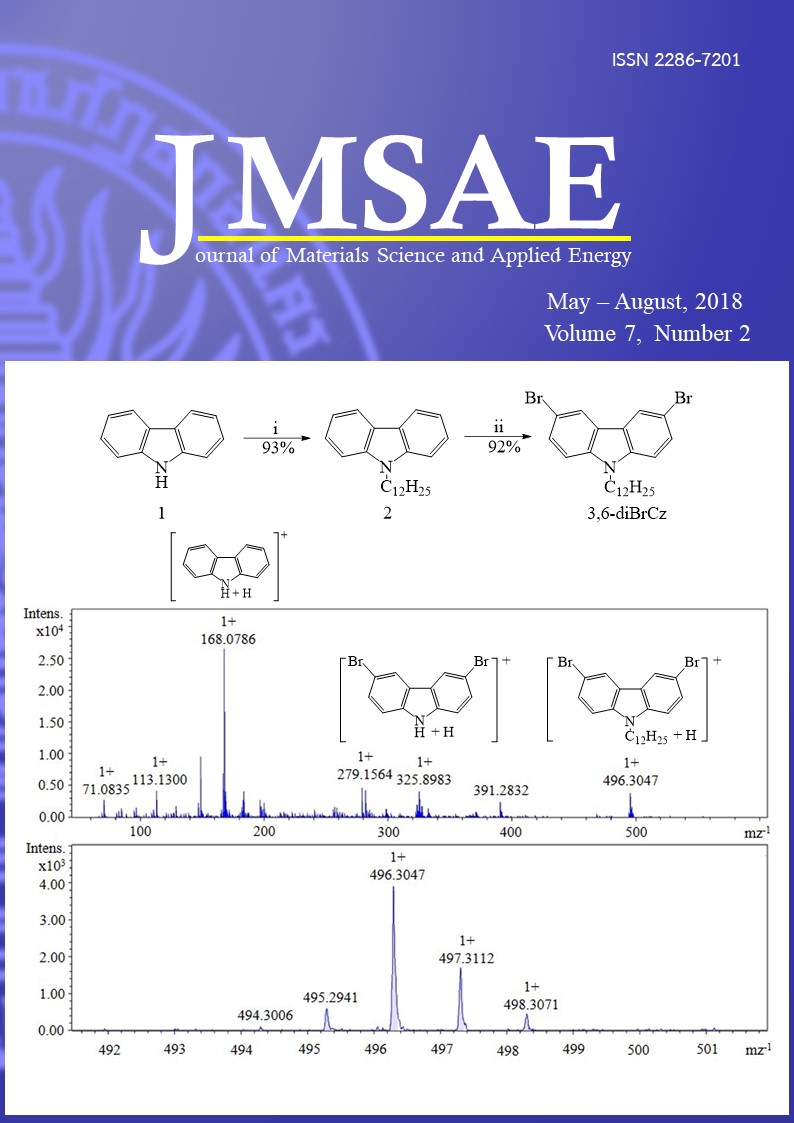
Isomeric 3,6-dibromo-9-dodecylcarbazole (3,6-diBrCz) and 2,7-dibromo-9-dodecylcarbazole (2,7-diBrCz) as a starting materials for conjugated materials were synthesized by Alkylation and Bromination reaction. This work reports a new and simple eco-friendly alkylation method for synthesis of 3,6-diBrCz and 2,7-diBrCz. All synthesized carbazole were purified by a silica gel with column chromatography, and were examined by 1H-NMR, 13C-NMR, FTIR, and DP-APCI-MS. Comparison of DP-APCI mass spectrum for 3,6-diBrCz and 2,7-diBrCz shows fragmentation routs are different. DP-APCI mass spectrum for 3,6-diBrCz shows the ions with mz-1 496.3047, 325.8983, and 168.0786 were assigned as C24H32Br2N+, C12H8Br2N+, and C12H10N+, respectively, in which firstly the dodecyl at N position and then bromine at C-3 and C-6 position were eliminated. DP-APCI mass spectrum for 2,7-diBrCz shows the ions with mz-1 496.3036, 336.2671, and 168.0789 were assigned as C24H32Br2N+, C24H34N+, and C12H10N+, respectively, in which firstly bromine at C-2 and C-7 position and then the dodecyl at N position were eliminated.