Annealing Induced Crystallinity, Photoelectrochemical Response and Hydrogen Evolution of TiO2 Nanotube Arrays as Photocatalyst in Water Splitting
DOI:
https://doi.org/10.55674/ias.v7i1.118869Keywords:
TiO2 nanotubes, crystallinity, water splitting, hydrogen evolutionAbstract
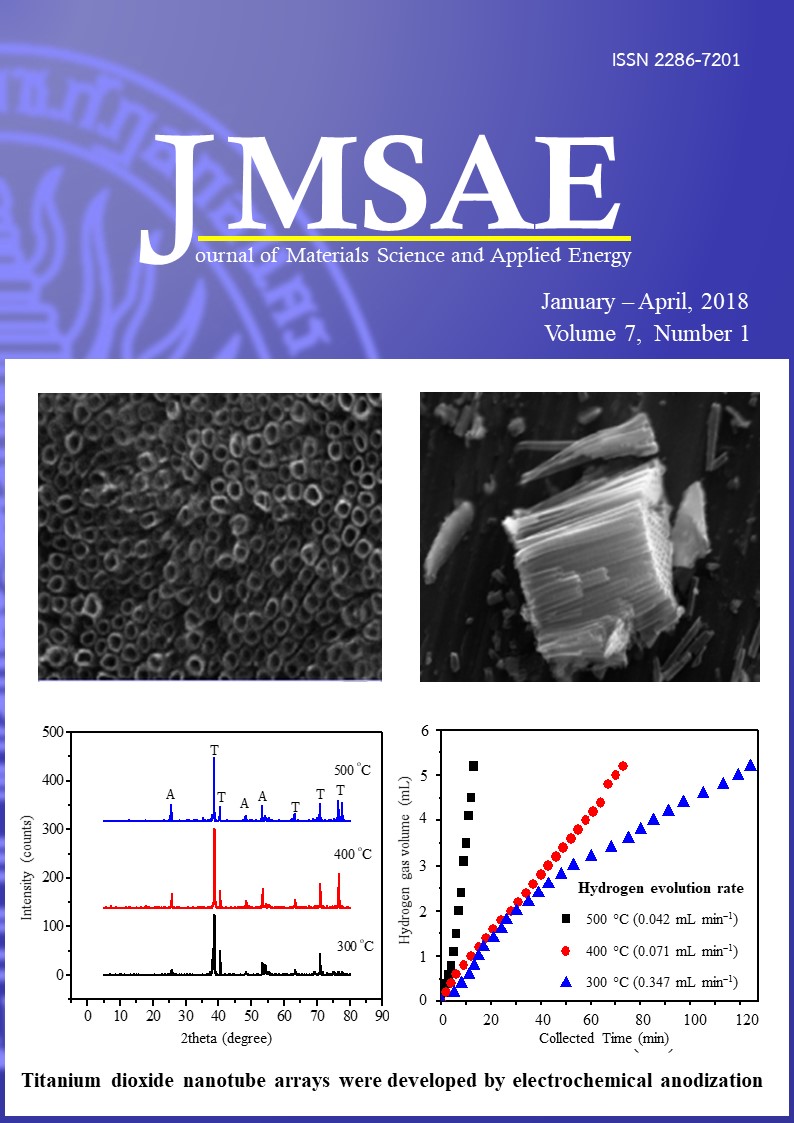
The titanium dioxide nanotube (TiO2-NT) arrays were developed by electrochemical anodization. Scanning electron microscopy investigation showed that the TiO2-NTs have well-defined morphology with average inner diameter of 100 nm and length 2mm. The crystallinity of anatase phase TiO2 is more pronounced at higher annealing temperature 500 ºC demonstrated by X-ray diffraction. We investigated the photoelectrochemical response of TiO2-NTs annealed at 300 ºC, 400ºC and 500ºC in KOH aqueous solution under light illumination with different wavelengths by linear sweep voltammetry. A significant increase in peak current densities is observed (i) under UV light and (ii) in TiO2-NTs annealed at 500 ºC. Former one is attributed to more energetic UV light activation to wide band gap TiO2 and latter one is ascribed to better crystallinity at higher temperature. In the photocatalytic water splitting experiment using white light illumination, hydrogen evolution rate increases with increasing annealing temperature of TiO2-NTs and is as high as 0.347mL/min for TiO2-NTs at 500 ºC. It is noted that the crystallinity of photocatalyst plays a key role in determining the electrochemical current generation and hydrogen evolution in photoelectrochemical water splitting.