Synthesis and Characterization of Novel di-Acceptor Carbazole Derivatives for Application to Dye Sensitized Solar Cells
DOI:
https://doi.org/10.55674/ias.v7i2.114697Keywords:
carbazole, fluorene, thiophene, donor-π-conjugated-acceptorAbstract
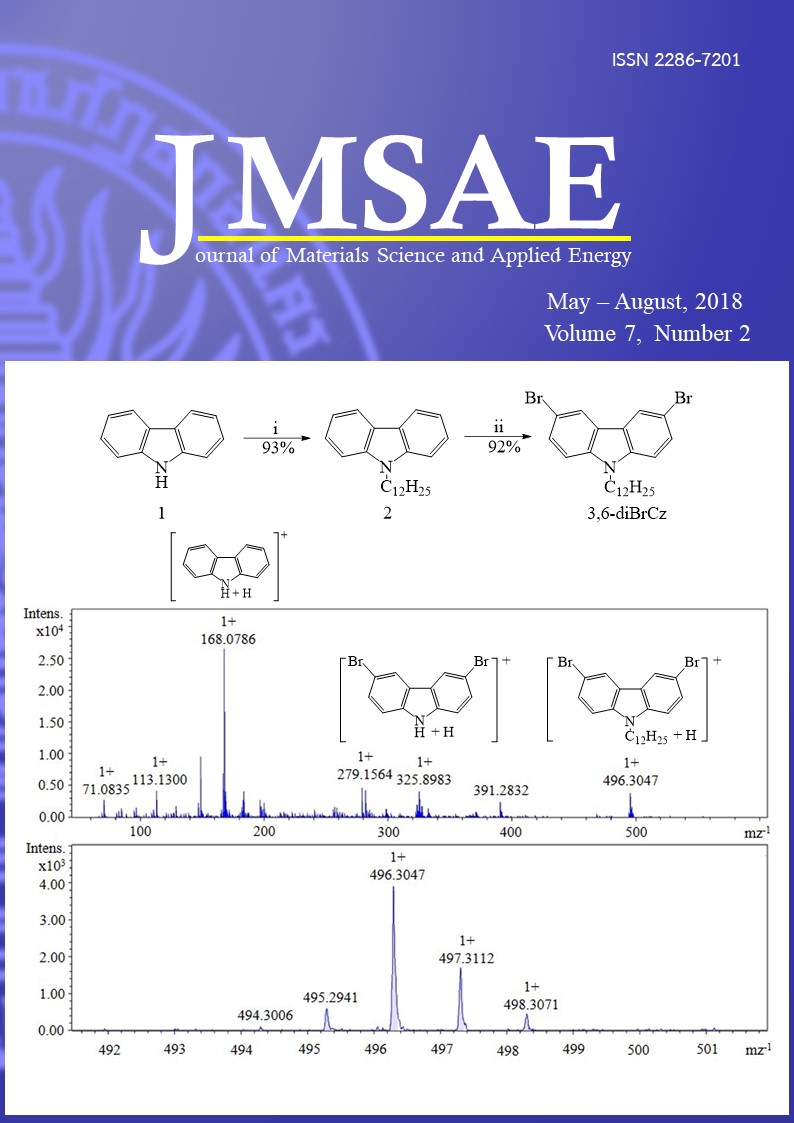
Novel three organic dyes with the general structure donor-(π-conjugated-acceptor)2 (D-(π-A)2) have been designed, synthesized and characterized for application to dye sensitized solar cells (DSSCs). The high soluble electron donor and acceptor groups of the dyes were carbazole and aldehyde moieties. The conjugated chain of the dyes contains a thiophene thiophene (TT) or thiophene phenyl (TP) unit. The introduction of a fluorene ring in the donor part of these dyes to increase of light harvesting, prevent dye aggregation and suppress the dark current significantly in dye-sensitized solar cells (DSSCs). The three bulky donor dyes (D1, D2 and D3), were successfully synthesized using bromination, alkylation, Ullmann coupling and Suzuki coupling reaction. The new divergent synthetic strategy was used to overcome the key bromination step. All of the novel target structures containing a bulky and high soluble carbazole group attached to a fluorene donor unit have been characterized by 1H-NMR, 13C-NMR, Mass spectrometry and FT-IR spectroscopy.