The relationship and impact of pH, ORP, and elemental contents on arsenic remediation by Cyperus papyrus (L.) under submerged soil conditions
Keywords:
Total Arsenic, Arsenite, Arsenate, Cyperus papyrus (L.), Submerged SoilAbstract
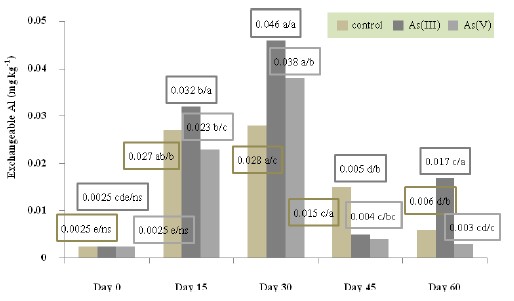
Arsenic remediation in wetlands is very important to the ecosystem. Phytoremediation efficiency is high and environment-friendly. Cyperus papyrus (L.) can highly selective accumulation arsenic from soil in large quantities. This objective study aimed to investigate variation of pH, redox potential, available phosphorus, extractable iron, exchangeable aluminum, exchangeable calcium and exchangeable magnesium for arsenic remediation in submerged soil by C. papyrus (L.). Experimental design was a 3 × 4 factorial design in a completely randomized experiment (CRD) included two factors. The first factor was the arsenic speciation consisting of arsenite and arsenate. The second factor was the cultivation periods of C. papyrus (L.) including 15, 30, 45 and 60 days. The result indicated that the mean comparison using DMRT of redox potential, total arsenic accumulation of C. papyrus (L.), exchangeable calcium and extractable iron were significantly different among all soil treatment groups (P-value < 0.01). The pH mean and exchangeable magnesium content were significantly different between arsenite and arsenate treated soil with control soil (P-value < 0.01 and 0.05). The amounts of available phosphorus and exchangeable aluminum were not significantly different among all soil types. The variation in group of three soil types during cultivation period 60 days showed that pH, redox potential, available phosphorus, extractable iron, exchangeable aluminum, exchangeable calcium and exchangeable magnesium were statistically significant (P-value < 0.01). The results of comparison among three treatment soils at each cultivating period demonstrated that redox potential, total arsenic accumulation, extractable iron, exchangeable aluminum, exchangeable calcium and exchangeable magnesium were significantly different among all cultivation periods (P-value < 0.01). pH was no significant difference on 15 days and available phosphorus was significant difference on 15 and 30 days (P-value < 0.01 and 0.05). Multiple regression analysis indicated that predictive factors of total arsenic accumulation of C. papyrus (L.) were arsenic speciation, redox potential, pH, and cultivation period. The model explained 85.2% in the regression model. The regression model was -339.542 + 20.249 (arsenic speciation) - 0.322 (Eh) + 52.681 (pH) – 0.987 (cultivating day).
References
Abdul, R., John, R., & George, E. (2001). Soil and plant laboratory manual, 2nded., Islamabad, Pakistan: NationalAgricultural Research Center.
Amita, J., Klaus, P. R., & Richard, H. L. (1999). Arsenite and arsenate adsorption on ferrihydrite: Surface charge reduction and net OH-release stoichiometry. Environmental Science & Technology, 33(8), 1179–1184. https://doi.org/10.1021/es980722e
Anderson, M. A., Ferguson, J. F., & Gavis, J. (1976). Arsenate adsorption on amorphous aluminum hydroxide. Journal of Colloid and Interface Science,54(3), 391-399. https://doi.org/10.1016/0021-9797(76)90318-0
American Water Work Association Water Environment Federation. (1998). Standard Methods for the Examination of Water and Wastewater, 20thed., New York, United states: American Public Health Association.
Bissen, M., & Frimmel, F.H. (2003). Arsenic-A review. Part I: Occurrence, toxicity, speciation, mobility. Acta hydrochim. Hydrobiol, 31(1), 9–18. https://doi.org/10.1002/aheh.200390025
Bolan, N., Mahimairaja, S., Kunhikrishnan, A., & Choppala, G. (2013). Phosphorus–arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Science of the Total Environment, 463, 1154–1162. https://doi.org/10.1016/j.scitotenv.2013.04.016
Bose, P., & Sharma, A. (2002). Role of iron in controlling speciation and mobilization of arsenic in subsurface environment. Water Research, 36, 4916–4926. https://doi.org/10.1016/S0043-1354(02)00203-8
Claes, B., Roger, H., Ingmar, P., & Maria, G. (2013). Plants influence on arsenic availability and speciation in the rhizosphere, roots and shoots of three different vegetables. Environmental Pollution, 184, 540-546. https://doi.org/10.1016/j.envpol.2013.10.003
Deok, H. M., Dimitris, D., & Nektaria, M. (2004). Arsenic immobilization by calcium -arsenic precipitates in lime treated soils. Science of the Total Environment,330, 171–185. https://doi.org/10.1016/j.scitotenv.2004.03.016
Ekkasit, A., Pornsawan, V., Kasem, C., and Patana, A. (2002). Selection of the emergent plants suitable for removal of arsenic from arsenic contaminated water[Master’s thesis]. Mahidol University. (in Thai)
Emil, A. C., Tiberiu, F., Michaela, P., Ioan, M., Bela, A., & Cecilia, R. (2006). Distribution study of inorganic arsenic (III) and (V) species in soil and their mobility in the area of Baia-Mare, Romania. Chemical Speciation and Bioavailability,18(1), 11-25. https://doi.org/10.3184/095422906782146294
Fuessle, R. W., & Taylor, M. A. (2004). Stabilization of arsenite wastes with prior oxidation. Journal of Environmental Engineering, 130(9), 1063–1066. https://doi.org/10.1061/(ASCE)0733-9372(2004)130:9(1063)
Gambrell, R. P., & Patrick Jr, W. H. (1989). Cu, Zn, and Cd availability in a sludge-amended soil under controlled pH and redox potential conditions. In Inorganic contaminants in the vadose zone (pp. 89-106). Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.10.1007/978-3-642-74451-8_7
Ghosh, B., Das, M. C., Gangopadhyay, A. K., Das, T. B., Singh, K., Lal, S., Mitra, S., Ansari, S. H., Goswami, T. K., Chakraborty, S. K., & Banerjee, N. N. (2003). Removal of arsenic from water by coagulation treatment using iron and magnesium salt. Indian Journal of Chemical Technology, 10, 87–95. http://nopr.niscpr.res.in/handle/123456789/22705
Goh, K. H., & Lim, T. T. (2005). Arsenic fractionation in a fine soil fraction and influence of various anions on its mobility in the subsurface environment. Applied geochemistry, 20(2), 229-239. https://doi.org/10.1016/j.apgeochem.2004.08.004
Islam, A., & Islam, W. (1973). Chemistry ofsubmerged soils and growth and yield of rice. Plant and soil, 39, 555 –565. https://doi.10.1007/BF00264174
Jain, A., Raven, K. P., & Loeppert, R. H. (1999). Arsenite and arsenate adsorption on ferrihydrite: surface charge reduction and net OH-release stoichiometry. Environmental Science & Technology, 33(8), 1179-1184. https://doi.org/10.1021/es980722e
Jomjun, N., Siripen, T., Jintapat, N., Maliwan, S., Prasak, T., and Somporn, C. (2018).Potential of Cyperus papyrus(L.) for Inorganic Arsenic Accumulation in Cases of Arsenite and Arsenate in Submerged Soil. Journal of Science Ladkrabang, 27(1), 1–17. https://www.thaiscience.info/Journals/Article/JOSL/10990044.pdf. (in Thai)
Kubicki, J. D. (2005). Comparison of As(III) and As(V) complexation onto Al-and Fe-hydroxides. Advances in Arsenic Research, 915, 104–117. https://doi.10.1021/bk-2005-0915.ch008
Lombi, E., Sletten, R. S. & Wenzel, W. W. (2000). Sequentially extracted arsenic from different size fractions of contaminated soils.Water, Air, & Soil Pollution,124, 319–332.https://doi.10.1023/A: 1005230628958
Lu, Y., Dong, F., Deacon, C., Chen, H. j., Raab, A., & Meharg, A. A. (2010). Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South China. Environmental Pollution,158(5), 1536–1541. https://doi.10.1016/j.envpol.2009.12.022
Mahajan, S., Pandey, G. K., & Tuteja, N. (2008). Calcium-and salt-stress signaling in plants: Shedding light on SOS pathway. Archives of Biochemistry and Biophysics,471(2), 146–158.https://doi.org/10.1016/ j.abb.2008.01.010
Masscheleyn, P. H., Dlaune, R. D., & Patrick, J. R. (1991). Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Science of the Total Environment, 25(8), 1414–1419. https://doi. org/10.1021/es00020a008
Massimo, P., Antonio, G. C., Lucia, C., Alessia, S., & Violante, A. (2015). Arsenic in the Soil Environment: Mobility and Phytoavailability. Environmental Engineering Science. 32(7), 551–563. https://doi. org/10.1089/ees.2015.0018
Nriagu, J. O., Bhattacharya, P., Mukherjee, A. B., Bundschuh, J., Zevenhoven, R., & Loeppert, R. H. (2007). Arsenic in soil and groundwater: an overview. Trace Metals and other Contaminants in the Environment, 9, 3-60.https://doi.org/10.1016/S1875-1121(06)09001-8
Petrusevski, B., Sharma, S., Schippers, J. C., & Shordt, K. (2007). Arsenic in Drinking Water, Thematic Overview. Oxford, United Kingdom: IRC International Water and Sanitation Centre.
Rahman, M. A., Hasegawa, H., Ueda, K., Maki, T., & Rahman, M. M. (2008). Arsenic uptake by aquatic macrophyte Spirodela polyrhizaL.: Interactions with phosphate and iron. Journal of Hazardous Materials, 160(2-3), 356-361.https://doi.org/10.1016/j.jhazmat.2008.03.022
Ravenscroft, P., Brammer, H., & Richards, K. (2009). Arsenic Pollution,New Jersey, United States: John Wiley & Sons.
Sanz, E., Munoz-Olivas, R., Camara, C., Sengupta, M. K., & Ahamed, S. (2007). Arsenic speciation in rice, straw, soil, hair and nails samples from the arsenic-affected areas of Middle and Lower Ganga plain. Journal of Environmental Science and Health,42(12), 1695–1705. https://doi.10.1080/10934520701564178
Senn, A. C., Hug, S. J., Kaegi, R., Hering, J. G., & Voegelin, A. (2018). Arsenate co-precipitation with Fe(II) oxidation products and retention or release during precipitate aging. Water Research, 131, 334–345. https://doi.org/10.1016/j.watres.2017.12.038
Signes-Pastor, A., Burlóa, F., Mitra, K., & Carbonell-Barrachina, A. A. (2007). Arsenic biogeochemistry as affected by phosphorus fertilizer addition, redox potential and pH in a west Bengal (India) soil. Geoderma,137, 504–510. https://doi.org/10.1016/j.geoderma.2006.10.012
Shree, J. D. (2018).Calcium (Ca): Forms, Sources and Behavior/Soil.Retrieved March 10, 2023, from http://www.soilmanagementindia.com.
Stevens, J. (1992).Applied multivariate statistics for the social sciences, 2nded., New Jersey, United States: Lawrance Erlbaum Associate, Inc.
Szegedi, K., Vetterlein, D., & Jahn, R. (2010). Modelling rhizosphere transport in the presence of goethite, including competitive uptake of phosphate and arsenate. Plant and Soil, 330(1), 481–501. https://doi.10. 1007/s11104-009-0221-9
Tingying, X. & Jeffrey, G. C. (2018). Effects of Ionic Strength on Arsenate Adsorption at Aluminum Hydroxide–Water Interfaces. Soil system. 2(1), 1 –13.https://doi.org/10.3390/soils2010001
Thor,K. (2019). Calcium-Nutrient and messenger. Frontiers in Plant Science,10, 440. https://doi. 10.3389/fpls.2019.00440
Violante, A., & Pigna, M. (200). Competitive sorption of arsenate and phosphate on different clay minerals and soils.Soil Science Society of America Journal,66, 1788–1796. https://doi.10.2136/sssaj2002.1788
Wei, L., Dehong, C., Fang, X., Jeannie, Z. Y. T., Pei-Pei, H., Wei-Guo, S., Natalita, M. N., & Rachel, A. C. (2016). Extremely high arsenic removal capacity for mesoporous aluminium magnesium oxide composites. Environmental Science: Nano,3(1), 94-106. https://doi.10.1039/C5EN00171D
Zeng, X., He, Q., Bai, L., Li, L., & Su, S. (2011). The arsenic speciation transformation in artificially arsenic-contaminated fluvo-aquic soil (Beijing, China). Plant, Soil and Environment,57(3), 108–114. https://doi.10.17221/198/2010-PSE
Zou, Q., Liu, F., & Yang, J. H. (2009). Adsorption-desorption and competitive adsorption of arsenic and phosphorus in purple soil. Journal of Applied Ecology,20(6), 1383–1389. http://www.cjae.net/EN/abstract/ abstract2339.shtml
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Journal of Applied Science and Emerging Technology

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

