Iron removal from synthetic aqueous solution using amino functionalized commercial silica gel as adsorbent
Keywords:
Amino functionalized commercial silica gel, Iron removal, adsorbentAbstract
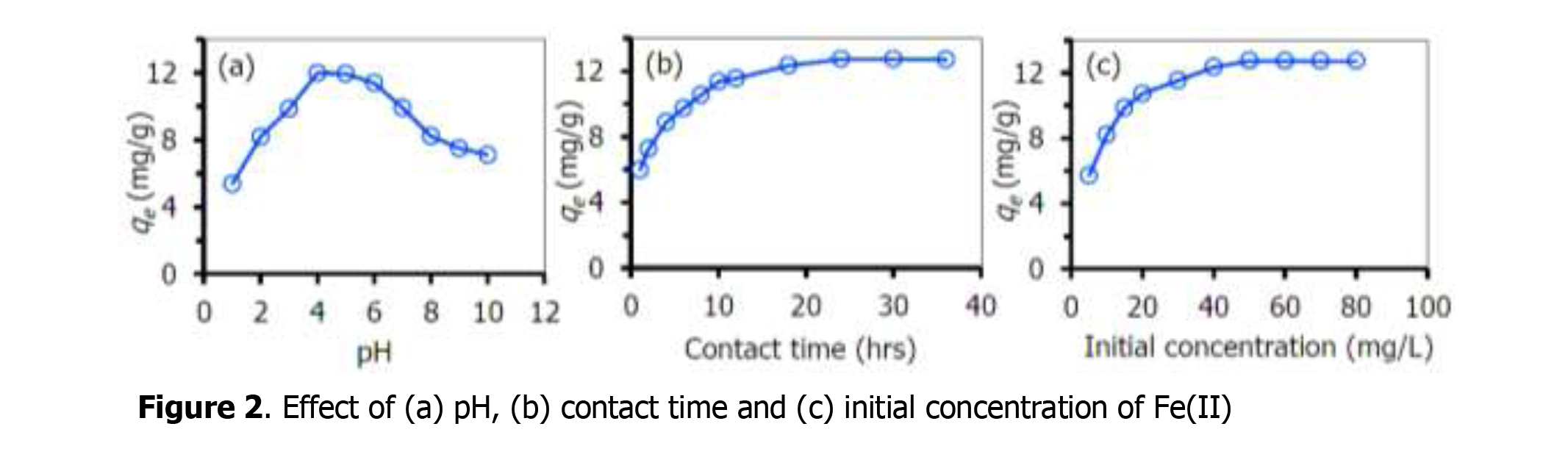
The amino group (-NH2) is one of the most important functional groups of ligands. Particularly, 3-aminopropyl-trimethoxysilane (APTMS) has relatively high affinity to bind various to metal ions. The present study was aimed to modify commercial silica gel (CSG) with the APTMS via silanization process to acquire amino groups (CSG-NH2) as an adsorbent for the removal of iron from synthetic aqueous solution. For an optimal adsorption study, the effects of the initial concentration of Fe(II) (1-80 mg/L), pH of solution (pH 1-10), contact time (1-36 hours) and temperature (30-60˚C) were investigated. The results showed that the adsorption capacity of the obtained CSG-NH2 for the Fe(II) was 60.80 mg/g at pH 4, and complete adsorption equilibrium was reached within 24 hrs. The adsorption isotherm of the CSG -NH2 for Fe(II) was well fitted by the Langmuir isotherm. In addition, thermodynamic data demonstrated that the Fe(II) adsorption onto the CSG-NH2 surface was mainly an exothermic spontaneous reaction. This implies that CSG-NH2 can be used as a high potential adsorbent for the removal of ferrous ion from contaminated wastewater.